From esters
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Aldehydes and Ketones
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

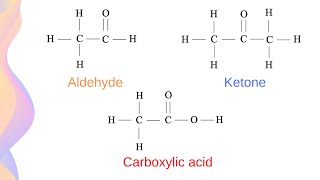
Today, we will introduce aldehydes and ketones. Can anyone tell me what a carbonyl group is? Remember, it has the structure >C=O.

Isn't it a functional group? I think it defines aldehydes and ketones.

Exactly! Aldehydes have a carbonyl group bonded to a hydrogen atom, while ketones have it bonded to two carbon atoms. Can anyone think of examples of each?

Formaldehyde is a common aldehyde, right?

And acetone is a ketone? It's used as a nail polish remover.

Correct! Remember, aldehydes and ketones play significant roles in our lives and industry. We'll explore their nomenclature next.
Nomenclature of Aldehydes and Ketones
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's delve into the nomenclature. For aldehydes, we use the suffix '-al'. Can someone give me an example?

How about ethanal for acetaldehyde?

Great! Now, ketones use the suffix '-one'. Let's discuss a ketone example.

Butan-2-one fits, right?

Exactly! Remember that the IUPAC names are structured based on the longest carbon chain. We'll summarize this in a table later.
Structure and Properties of Carbonyl Group
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, what can you tell me about the structure of the carbonyl group?

I think it's trigonal planar with bond angles around 120 degrees.

Exactly! This structure leads to polarity. How do you think this affects its physical properties?

Maybe it means that they have higher boiling points than similar hydrocarbons?

Right again! Their higher boiling points arise from dipole-dipole interactions compared to ethers and hydrocarbons.
Preparation of Aldehydes and Ketones
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s talk about how we can prepare these compounds. How can primary alcohols be converted?

They can be oxidized to create aldehydes.

Correct! And what about secondary alcohols?

Those turn into ketones when oxidized.

Perfect! Can anyone identify a reaction type that yields both?

Ozonolysis of alkenes can give us aldehydes and ketones.

Exactly! When we study more reactions, pay attention to specifics like conditions and reagents.
Applications of Aldehydes and Ketones
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Lastly, let’s discuss where we find aldehydes and ketones in everyday life. Where can they be used?

I know that they help in perfumes and flavoring!

Exactly! They enhance fragrances in personal care products. Any other uses?

Like in making plastics and solvents!

Absolutely! Aldehydes and ketones are versatile. The final takeaway is their importance in industrial and pharmaceutical applications.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section focuses on the fundamental concepts related to aldehydes, ketones, and carboxylic acids, including their functional groups, common and IUPAC nomenclature, methods of preparation, and reactions. It also highlights their importance in various biological and industrial applications.
Detailed
Understanding Aldehydes, Ketones, and Carboxylic Acids
This section explores carbonyl compounds, specifically aldehydes, ketones, and carboxylic acids, which are crucial in organic chemistry and various practical applications, including food products and pharmaceuticals. The carbonyl group can be categorized primarily into aldehydes (which contain at least one hydrogen attached to the carbonyl) and ketones (with two carbon groups attached). The distinction in nomenclature, functional group structure, and importance of these compounds is articulated through their uses in everyday items and biological processes.
Nomenclature Overview
- Common Names: Derived from acids for aldehydes, indicating the source or structures. For ketones, it involves naming the carbon chains involved.
- IUPAC Names: For aldehydes, the suffix ‘-al’ is used, and for ketones, ‘-one’ is applied, following standardized rules for naming based on the carbon chain length and structure.
Preparation Techniques
- Aldehydes: Commonly synthesized through the oxidation of primary alcohols or other chemical reactions, including side-chain oxidation of aromatic compounds.
- Ketones: Prepared by oxidizing secondary alcohols or from acyl halides through Friedel-Crafts acylation.
Understanding these compounds is pivotal due to their extensive applicability in the production of perfumes, flavoring agents, and solvents, as well as in biochemical processes. The section imparts essential knowledge necessary to comprehend advanced organic chemistry topics and molecular interactions.
Youtube Videos









Key Concepts
-
Functional Groups: Aldehydes and ketones are defined by their carbonyl groups.
-
Nomenclature: Proper naming conventions are critical for identifying these compounds.
-
Reactivity: Carbonyl compounds exhibit distinct reactivity patterns based on their structure.
Examples & Applications
Formaldehyde (HCHO) is a vital preservative.
Acetone ((CH3)2CO) is commonly used as a solvent in nail polish removers.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Always donate H with aldehydes, Ketones keep it doubled with their carbon sides.
Stories
Imagine a chef using aldehydes to enhance flavors—like adding vanilla extract (an aldehyde) for its aroma in desserts, while using acetone in drinks as a stabilizer (a ketone).
Memory Tools
Aldehydes can have one hydrogen, think 'A' for one. Ketones have two carbon chains, 'K' for two.
Acronyms
Remember 'AC' stands for Aldehyde and Carbonyl for the essential team's presence (A for Aldehyde, C for Carbonyl).
Flash Cards
Glossary
- Carbonyl Group
A functional group characterized by a carbon atom double-bonded to an oxygen atom (>C=O).
- Aldehyde
Organic compounds with a carbonyl group bonded to at least one hydrogen atom.
- Ketone
Organic compounds with a carbonyl group bonded to two carbon atoms.
- IUPAC Nomenclature
A systematic method of naming organic chemical compounds based on their structure.
Reference links
Supplementary resources to enhance your learning experience.
