Carbylamine reaction
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Carbylamine Reaction
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we will discuss the carbylamine reaction. Can anyone tell me what this reaction is about?

Is it related to how amines react with chloroform?

Exactly! The carbylamine reaction involves primary amines reacting with chloroform and alcoholic potassium hydroxide to produce isocyanides. It's an important test for primary amines.

What are isocyanides?

Isocyanides, or carbylamines, are compounds characterized by a foul smell. This is a key feature that helps to identify the reaction.

What happens with secondary and tertiary amines?

Good question! Secondary and tertiary amines do not react in this way. So, the carbylamine reaction is specific to primary amines.

Let's summarize: the carbylamine reaction is primarily a qualitative test for primary amines, yielding foul-smelling isocyanides.
Chemical Equation of Carbylamine Reaction
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's look at the chemical equation for the carbylamine reaction. Can anyone write it down?

Is it R-NH₂ + CHCl₃ + 3KOH → R-NC + 3KCl + 3H₂O?

That's correct! Each component plays a crucial role. Could someone explain what 'R' stands for in this equation?

R represents the alkyl or aryl group attached to the amine.

Right! This means the reaction can occur with various primary amines, both aliphatic and aromatic.

Summarizing, the carbylamine reaction helps us identify primary amines through its distinct products.
Practical Applications of Carbylamine Reaction
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

What do you think are some applications of the carbylamine reaction?

It helps test for primary amines in substances, right?

Exactly! It's widely used in laboratories for qualitative analysis. Can anyone think of more applications?

Maybe in organic synthesis?

Yes! Understanding this reaction assists in synthesizing various organic compounds. It highlights the reactivity patterns of amines.

In summary, the carbylamine reaction not only serves as a test but also plays a crucial role in synthesis and analysis in organic chemistry.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
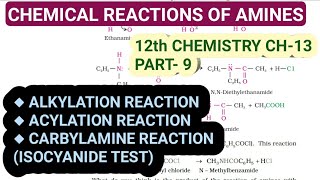
In the carbylamine reaction, aliphatic and aromatic primary amines react with chloroform and ethanolic potassium hydroxide to yield isocyanides, which have a distinct foul odor. This reaction is distinctive as secondary and tertiary amines do not participate, making it a specific test for primary amines.
Detailed
Carbylamine Reaction
The carbylamine reaction, also known as the isocyanide test, involves the reaction of aliphatic and aromatic primary amines with chloroform (
CCl₃) in the presence of ethanolic potassium hydroxide (KOH). Upon heating, this reaction produces isocyanides (or carbylamines), substances characterized by their strong and offensive odors. The overall chemical process can be represented by the equation:
R-NH₂ + CHCl₃ + 3KOH → R-NC + 3KCl + 3H₂O
This reaction highlights an important distinction in organic chemistry: secondary and tertiary amines do not undergo the carbylamine reaction. As a result, the reaction serves as a useful qualitative test for the presence of primary amines, which are commonly found in various biological and synthetic compounds. Understanding the carbylamine reaction provides significant insight into the reactivity of amines and can aid in the identification and analysis of organic substances.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Carbylamine Reaction
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Aliphatic and aromatic primary amines on heating with chloroform and ethanolic potassium hydroxide form isocyanides or carbylamines which are foul smelling substances.
Detailed Explanation
The carbylamine reaction is a significant chemical test that occurs when primary amines react with chloroform (CHCl3) and ethanolic potassium hydroxide (KOH). During this reaction, the primary amines undergo a transformation to form isocyanides (also known as carbylamines). Isocyanides are characterized by their foul smell, which serves as a key indicator to confirm the presence of primary amines.
Examples & Analogies
Think of the carbylamine reaction as a cooking process where the wrong ingredient is added, resulting in an unpleasant dish. Similarly, mixing chloroform with the right primary amine produces a distinctly unpleasant odor, signaling successful identification of the amine.
Mechanism of the Carbylamine Reaction
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Secondary and tertiary amines do not show this reaction. This reaction is known as carbylamine reaction or isocyanide test and is used as a test for primary amines.
Detailed Explanation
One of the crucial aspects of the carbylamine reaction is that it specifically occurs with primary amines—those containing one amino group attached to one carbon atom. Secondary and tertiary amines do not react in this manner because they lack the necessary structural components to form isocyanides. This specificity is what makes the carbylamine reaction a reliable test for identifying primary amines.
Examples & Analogies
Imagine you are testing for a specific type of fruit in a basket. If you only look for apples (representing primary amines), you will pass over oranges and bananas (representing secondary and tertiary amines) which do not give you the same result.
Applications of the Carbylamine Reaction
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The carbylamine reaction is used as a test for primary amines.
Detailed Explanation
The ability to identify primary amines quickly and effectively using the carbylamine reaction is immensely valuable in organic chemistry. This method provides a straightforward approach to confirm the presence of primary amines in various chemical compounds, enhancing our understanding and manipulation of organic substances.
Examples & Analogies
Consider the carbylamine reaction as a detective having a signature method to identify a suspect in a crowd. The distinct smell of the isocyanide acts as the 'fingerprint' uniquely pointing to the presence of a primary amine, just as the detective uses unique clues to confirm the identity of the suspect.
Key Concepts
-
Carbylamine Reaction: A test for the presence of primary amines using chloroform and potassium hydroxide.
-
Isocyanides: The foul-smelling products formed from the carbylamine reaction.
-
Primary Amines: Compounds capable of undergoing the carbylamine reaction; characterized by one alkyl or aryl group.
Examples & Applications
Example 1: Heating an aliphatic primary amine like ethylamine with chloroform and potassium hydroxide would yield ethyl isocyanide.
Example 2: Aromatic primary amines such as aniline would react similarly to produce phenyl isocyanide.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In the carbylamine’s heat and smoke, smelling bad, is no joke.
Stories
Once in a lab, a chemist heated primary amines with chloroform. The resulting foul-smelling isocyanide became the talk of the lab; everyone learned that only primary amines could create this stinky compound.
Memory Tools
Remember 'CARB' for the carbylamine reaction: C for Chloroform, A for Alcoholic KOH, R for Reactions of primary amines, and B for the Bad smell of isocyanides.
Acronyms
Remember the acronym P.A. for primary amines that play a role in the carbylamine reaction.
Flash Cards
Glossary
- Carbylamine
A compound formed from primary amines and chloroform in the presence of potassium hydroxide, characterized by a foul smell.
- Isocyanide
A type of organic compound featuring the isocyanide functional group (-N≡C), derived from the carbylamine reaction.
- Primary amine
An amine in which one alkyl or aryl group is attached to the nitrogen atom.
- Secondary amine
An amine with two alkyl or aryl groups attached to the nitrogen atom, which does not react in the carbylamine reaction.
- Tertiary amine
An amine with three alkyl or aryl groups attached to the nitrogen atom, which also does not participate in the carbylamine reaction.
Reference links
Supplementary resources to enhance your learning experience.
