A1.1 Water
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Molecular Structure of Water
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we start with the fundamental molecule of life: water. Each water molecule consists of one oxygen atom and two hydrogen atoms bonded together. Can anyone tell me what kind of bonds hold these atoms together?

Covalent bonds? That’s where the electrons are shared, right?

Exactly! This sharing forms a polar molecule because the oxygen atom is more electronegative than hydrogen. This creates partial charges. What's important about this polarity?

It helps water form hydrogen bonds with itself and other molecules!

Great observation! Each water molecule can form up to four hydrogen bonds. This property is what leads to water's cohesion, which is essential for many biological functions.

How does cohesion help plants specifically?

Excellent question! Cohesion in water creates surface tension, which helps water move from roots to leaves through processes like transpiration.

To sum up, the polar nature of water and its ability to form hydrogen bonds are key to its unique properties that support life.
Hydrogen Bonding and Physical Properties
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Continuing from our last session, let’s dive into how hydrogen bonding affects water's physical properties. Can anyone recall what cohesion and adhesion mean?

Cohesion is when water molecules stick to each other, and adhesion is when they stick to other surfaces.

Exactly! Cohesion contributes to surface tension, while adhesion allows water to move against gravity in plant xylem. What do you think happens when we place a straw in a glass of water?

The water goes up the straw due to capillary action, right?

Right again! That’s an excellent example of adhesion and cohesion in action. This property is crucial for transporting water and nutrients in plants.

What about water's high specific heat? How does that help organisms?

Water's high specific heat means it can absorb a lot of heat without changing temperature drastically, which helps maintain stable environments for aquatic organisms and regulates body temperature in endothermic animals.

To recap, hydrogen bonding gives water unique properties like cohesion, adhesion, and a high specific heat capacity, all vital for supporting life.
Water's Role in Biological Systems
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s discuss how water functions in biological systems. What roles do you think water plays in organisms?

It’s involved in transporting nutrients and waste in blood or sap!

Correct! Water is the primary component of blood, which transports oxygen, nutrients, and waste products. What else?

It's involved in metabolic reactions, right? Like hydrolysis and condensation?

Exactly! Water participates in hydrolysis reactions, breaking down macromolecules, and is produced in condensation reactions when forming polymers. How about temperature regulation?

The specific heat of water helps maintain stable temperatures within organisms.

Spot on! And let's not forget the structural support water provides to plants through turgor pressure. Who can remind me how this works?

Water fills the central vacuole, creating pressure against the cell wall, which keeps the plant upright.

Great summary! Water is essential not only for chemical reactions but also for transport, temperature regulation, and providing structural support.
High Latent Heat of Vaporization and Density Anomaly
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

In this session, we focus on two more unique properties of water: high latent heat of vaporization and density anomaly. What does high latent heat of vaporization mean for us?

It means water needs a lot of energy to change from liquid to vapor, which helps cool things down!

Exactly! This means sweating or panting can effectively cool organisms, helping with thermoregulation. Now, let’s talk about the density anomaly. How does this property affect aquatic life?

Ice floats on water, creating insulation for aquatic organisms in winter!

Perfect! As water freezes, it becomes less dense and forms ice, providing a protective layer for organisms below. Can someone summarize why these properties are critical for life on Earth?

They help regulate temperature and provide a stable environment for life!

Correct! Water's unique properties are vital for maintaining life as we know it.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Water's molecular structure gives it unique properties such as high cohesion and adhesion, which are crucial for biological processes. These properties impact temperature regulation, transport mechanisms, and metabolic reactions in living organisms.
Detailed
A1.1 Water
Water (H₂O) is essential for life and exhibits unique properties attributed to its molecular structure. A water molecule comprises one oxygen atom covalently bonded to two hydrogen atoms, creating a polar molecule that forms hydrogen bonds with adjacent water molecules. This leads to several key properties:
- Cohesion: Water molecules tend to stick together due to hydrogen bonding, leading to high surface tension, which is crucial for processes like transpiration in plants.
- Adhesion: The attraction between water molecules and other surfaces enhances water's ability to move through narrow spaces, contributing to capillary action.
- High Specific Heat Capacity: Water can absorb significant heat without drastic temperature changes, stabilizing environments for aquatic life.
- Latent Heat of Vaporization: High energy is required for water to evaporate, aiding in thermoregulation through sweating in animals.
- Density Anomaly: Water is less dense as ice than as a liquid, allowing ice to float and insulating aquatic ecosystems.
- Universal Solvent: Water's polarity makes it an excellent solvent for a variety of substances, facilitating biochemical reactions and transport within living organisms.
These properties underscore water's integral role as a transport medium in animals and plants, its participation in metabolic reactions, and its regulatory functions in maintaining homeostasis.
Youtube Videos
Audio Book
Dive deep into the subject with an immersive audiobook experience.
Molecular Structure of Water
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
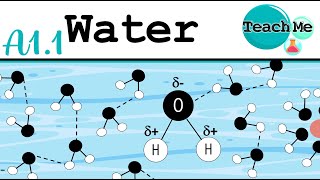
● A water (H₂O) molecule consists of one oxygen atom covalently bonded to two hydrogen atoms at an angle of approximately 104.5°.
● Oxygen’s high electronegativity draws shared electrons closer, producing a partial negative charge (δ–) on the oxygen atom and partial positive charges (δ+) on the hydrogen atoms.
● This polar geometry allows each water molecule to form up to four hydrogen bonds (two through its hydrogen atoms, two through lone pairs on oxygen) with neighboring water molecules.
Detailed Explanation
Water consists of one oxygen atom bonded to two hydrogen atoms, creating a bent shape due to the angle of about 104.5 degrees. Because oxygen attracts electrons more strongly than hydrogen, it carries a slight negative charge while the hydrogen atoms carry slight positive charges. This uneven charge distribution makes water a polar molecule, which is crucial for its ability to form hydrogen bonds with other water molecules, allowing for multiple interactions that stabilize its structure.
Examples & Analogies
Think of water molecules as tiny magnets. The oxygen part is like the negative end of a magnet that attracts the hydrogen parts (the positive ends of other magnets), allowing them to stick together. This is why you can see water droplets form; they are clumping together due to these attractions.
Hydrogen Bonding and Physical Properties
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
-
Cohesion and Surface Tension
○ Hydrogen bonds between water molecules generate strong cohesive forces, causing water to “stick” to itself.
○ At the air–water interface, surface water molecules bond more strongly to molecules beneath them than to air, resulting in a “skin-like” surface known as surface tension.
○ Biological relevance: In plants, cohesion contributes to the formation of a continuous column of water from roots to leaves (transpiration stream). -
Adhesion and Capillarity
○ Adhesion is the attraction between water molecules and other polar or charged surfaces (e.g., cellulose fibers in xylem).
○ Capillary action arises when adhesive forces between water and a narrow tube’s walls (or plant xylem) exceed cohesive forces, enabling water to climb against gravity. -
High Specific Heat Capacity
○ Water can absorb or release large amounts of heat with minimal change in temperature.
○ This high specific heat capacity (4.18 J·g⁻¹·°C⁻¹) stabilizes aquatic environments and moderates climate, buffering temperature fluctuations.
○ Biological relevance: Aquatic organisms experience relatively constant temperatures; endothermic animals maintain stable internal temperatures.
Detailed Explanation
Water molecules hold tightly to one another (cohesion), which creates surface tension; this is like having a thin 'skin' on the surface of the water. Additionally, when water interacts with other surfaces (adhesion), it can move up through small tubes (capillarity), assisting plants in transporting water from their roots to leaves. Water's high specific heat capacity means it can absorb a lot of heat without changing temperature significantly, helping to regulate environments and living organisms that require stable conditions.
Examples & Analogies
If you've ever seen a bug walking on water, that's due to cohesion creating surface tension. If you've used a straw to drink, you experienced capillary action. When you heat water, it takes quite a bit of energy to change its temperature, like how a heavy blanket keeps you warm without overheating; it holds that warmth well.
High Latent Heat of Vaporization
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
○ To convert 1 g of liquid water at 100 °C into vapor, about 2260 J of energy is required (latent heat of vaporization).
○ Evaporation from body surfaces (sweating, panting) removes substantial heat, aiding thermoregulation.
Detailed Explanation
Water requires a significant amount of energy to change from liquid to vapor, specifically 2260 joules for just 1 gram at boiling point. This process is known as the latent heat of vaporization. This property is vital for living organisms as it allows for effective cooling through evaporation (like sweating), which helps maintain a stable body temperature.
Examples & Analogies
Think of sweating as nature's air conditioning. When you sweat, your body uses energy to turn the sweat into vapor, and as it evaporates, it cools your skin, much like having a fan cool you off after you've had a workout.
Density Anomaly and Ice Floating
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
○ As water cools below 4 °C, its density decreases; ice (0 °C) is about 9% less dense than liquid water.
○ Ice floats, creating an insulating layer on lakes and ponds that protects aquatic life in winter.
Detailed Explanation
Unlike most substances, water expands and becomes less dense when it freezes, meaning ice is lighter than liquid water. This is crucial because it allows ice to float on the surface, forming an insulating layer that protects the water below from freezing, ensuring that aquatic life can survive beneath the ice during winter.
Examples & Analogies
Picture how a cooler stays insulated with ice. The layer of ice on top of a pond works similarly; it acts like a barrier that keeps the water below warmer, allowing fish to continue swimming underneath, rather than freezing solid like the ice above.
Universal Solvent
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
○ Water’s polarity allows it to surround and dissolve ionic compounds (e.g., NaCl) by orienting the negative dipoles toward cations and positive dipoles toward anions.
○ Polar organic molecules (e.g., sugars, amino acids) also dissolve readily, facilitating transport and metabolic reactions.
Detailed Explanation
Water is often called the 'universal solvent' because its polar nature enables it to dissolve many substances, including ionic compounds like salt. The positive hydrogen ends of water molecules attract negative ions (anions), and the negative oxygen end attracts positive ions (cations), allowing them to separate and disperse in solution. This property is essential for biological processes, as many reactions occur in aqueous environments where substances need to be dissolved.
Examples & Analogies
When you add salt to water, it disappears. Imagine water molecules as tiny bus riders that pick up each salt ion, transporting them away so they can mingle with other substances, similar to how a crowd of people breaks apart and moves after a concert.
Water in Biological Systems
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
-
Transport Medium
○ In animals: Blood (mostly water) carries nutrients, gases, hormones, and waste products.
○ In plants: Sap (xylem and phloem fluids) is water-based, transporting minerals and organic solutes to all tissues. -
Metabolic Reactions
○ Water participates in hydrolysis reactions (cleavage of bonds by adding water) during digestion of macromolecules (e.g., proteins → amino acids, polysaccharides → monosaccharides).
○ In condensation (dehydration synthesis) reactions, water is produced when monomers join to form polymers (e.g., amino acids → polypeptides, nucleotides → nucleic acids). -
Temperature Regulation and Homeostasis
○ High specific heat capacity helps maintain stable internal body temperatures in homeotherms (e.g., mammals, birds).
○ Evaporative cooling (sweating, panting) exploits water’s high latent heat of vaporization.
Detailed Explanation
Water serves multiple critical roles in biological systems. It acts as a transport medium for vital substances (like blood and plant sap), is involved in metabolic reactions (such as breaking down or building up macromolecules), and aids in temperature regulation (keeping organisms' internal environments stable). This highlights the significance of water for sustaining life and facilitating various biological processes.
Examples & Analogies
Think of blood in your veins like a delivery truck, moving essential packages (nutrients, oxygen) around your body—water is vital for this transport. When you sweat, it’s like turning on a cooling system for your body, preventing overheating during hot days or after exercising.
Key Concepts
-
Molecular Structure of Water: Water consists of one oxygen atom and two hydrogen atoms, forming a polar molecule.
-
Hydrogen Bonding: Water's hydrogen bonds lead to properties like cohesion and adhesion.
-
High Specific Heat Capacity: Water can absorb significant heat without changing temperature rapidly.
-
High Latent Heat of Vaporization: Water requires substantial energy to evaporate, aiding in cooling processes.
-
Density Anomaly: Ice is less dense than liquid water, allowing it to float and insulate aquatic habitats.
-
Universal Solvent: Water dissolves many substances, essential for chemical reactions in biological systems.
Examples & Applications
Cohesion helps water move up through plant xylem, enabling nutrient transport.
Water's high specific heat stabilizes ocean temperatures, supporting marine life.
Ice floating on lakes prevents them from freezing solid, protecting aquatic ecosystems.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Water's great, it cools and flows, Keeps our bodies where life grows.
Stories
Picture a plant yearning to drink from deep roots, rising up through the xylem, sticking together like best friends—water moves upward magically with them, showing us the strength of togetherness through cohesion.
Memory Tools
Remember the acronym 'CATS' for water properties: Cohesion, Adhesion, Temperature stability, Solvent.
Acronyms
W.A.T.E.R
'Wonderful And True Essential Resource'
Flash Cards
Glossary
- Cohesion
The attraction between molecules of the same substance, leading to the formation of a solid or liquid.
- Adhesion
The attraction between molecules of different substances, which facilitates capillary action.
- High Specific Heat Capacity
The amount of heat required to change the temperature of water, contributing to temperature stability.
- Latent Heat of Vaporization
The energy required for water to change from liquid to vapor; important for cooling mechanisms in organisms.
- Density Anomaly
The unique property of water that allows it to be less dense as ice than as a liquid, enabling ice to float.
- Universal Solvent
Water’s ability to dissolve many substances due to its polarity, facilitating biochemical reactions.
Reference links
Supplementary resources to enhance your learning experience.
