What are Redox Reactions?
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Redox Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Good morning, everyone! Today, we will discuss redox reactions. Can anyone tell me what a redox reaction is?

Is it when one substance is oxidized and another is reduced?

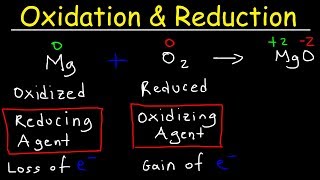
Exactly! A redox reaction involves the transfer of electrons between substances. Remember, oxidation means losing electrons, and reduction means gaining them. A handy way to remember this is with the mnemonic OIL RIG — Oxidation Is Loss, Reduction Is Gain.

What do you mean by losing and gaining electrons?

Great question! When a substance loses electrons, its oxidation state increases, meaning it is oxidized. Conversely, when it gains electrons, its oxidation state decreases, indicating that it is reduced.

Can you give us an example of a redox reaction?

Sure, consider the reaction between zinc and copper sulfate. Zinc is oxidized from 0 to +2, and copper is reduced from +2 to 0. We'll explore this more soon.

So, both processes happen at the same time, right?

Correct! Oxidation and reduction always happen together in redox reactions.

To wrap up, what are the key points we learned today?

Redox reactions involve electron transfer, with oxidation being the loss of electrons and reduction being the gain.
Oxidation States
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand redox reactions, let’s talk about oxidation states. Why do you think tracking oxidation states is important?

To know how many electrons an atom has?

Exactly! The oxidation state tells us how many electrons an atom has gained or lost. Here’s a key rule: the oxidation state of an atom in elemental form is always 0. For example, in oxygen gas, O₂, each oxygen atom has an oxidation state of 0.

What about in compounds?

In compounds, oxygen usually has an oxidation state of -2, while hydrogen is often +1. Here’s a great example: in water, H₂O, the oxidation states are +1 for hydrogen and -2 for oxygen.

Why is it always 0 in elemental form?

Because in elemental forms, atoms are not bonded to other elements and have not lost or gained electrons. Now, can anyone tell me what the sum of oxidation states in a neutral compound must be?

It must be zero!

Correct! And in polyatomic ions, the sum equals the ion's charge.
Identifying Oxidation and Reduction
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let’s see how we can identify oxidation and reduction in reactions. First, what’s the first step?

Assign oxidation numbers to each element?

Yes! Once we assign oxidation numbers, we can determine any changes. If an oxidation number increases, that element is oxidized. And if it decreases, that element is reduced. Let's look again at our zinc and copper sulfate example.

In that reaction, zinc went from 0 to +2, so it's oxidized.

Right! And what about copper?

Copper went from +2 to 0, which means it's reduced.

Awesome! Understanding these changes in oxidation states is crucial for recognizing redox reactions.
Balancing Redox Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Another important aspect of redox reactions is balancing them. Can anyone tell me why balancing is necessary?

To make sure both mass and charge are the same on both sides?

Exactly! We can use the half-reaction method or the ion-electron method to balance redox reactions. Who can summarize the half-reaction method?

First, write the oxidation and reduction half-reactions, then balance all other elements, and use H₂O and H⁺ or OH⁻ to balance oxygen and hydrogen.

Right! Finally, balance the charges with electrons. Let’s practice this with an example after class.
Applications of Redox Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let’s discuss how redox reactions are applied in the real world. Can anyone give examples of such reactions?

Rusting is a redox reaction, right?

Absolutely! Rusting involves iron being oxidized while oxygen is reduced. What about batteries?

Batteries use redox reactions to generate electrical energy!

Correct! And think of photosynthesis and respiration, where glucose and oxygen undergo redox processes as well. See how these concepts tie together?
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Youtube Videos




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Redox Reactions
Chapter 1 of 1
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A redox reaction is a chemical process where one substance gets oxidized (loses electrons) and another gets reduced (gains electrons). These reactions always occur simultaneously because for one species to lose electrons, another must gain them.
Detailed Explanation
A redox reaction involves a transfer of electrons between two substances. When one substance loses electrons, it undergoes oxidation. Conversely, when another substance gains those electrons, it undergoes reduction. This means redox reactions are fundamentally about how substances interact through the gain or loss of electrons, and because these processes are linked, they always happen together.
Examples & Analogies
Think of a game where one player passes a ball to another. The player losing the ball is like the one getting oxidized, while the player who receives the ball is like the one getting reduced. Just like in the game, where the player cannot lose the ball without someone else receiving it, electrons cannot be lost without another substance gaining them.
Key Concepts
-
Redox Definitions:
-
Oxidation: Loss of electrons by a molecule, atom, or ion.
-
Reduction: Gain of electrons by a molecule, atom, or ion.
-
Mnemonic: Remember 'OIL RIG' – Oxidation Is Loss, Reduction Is Gain.
-
Oxidation States: The oxidation state tracks electron changes during reactions, with essential rules for determining these states. For instance, elemental forms have an oxidation state of 0, oxygen usually is -2, and hydrogen is +1.
-
Identifying Changes: To classify elements in oxidation and reduction, assign oxidation numbers and note their changes during the reaction.
-
Half-Reactions: Redox reactions can be split into oxidation and reduction half-reactions, which help visualize the electron transfer.
-
Balancing: It is crucial to balance mass and charge in redox reactions using methods like the half-reaction method and the ion-electron method.
-
Applications: Redox reactions are crucial in many processes—corrosion (rusting), batteries (electrical energy), photosynthesis, and respiration are all explained through redox principles.
-
Importance
-
Mastering the concepts of redox reactions is foundational for students to understand various chemical reactions and their importance in both natural and industrial processes.
Examples & Applications
When zinc (Zn) reacts with copper sulfate (CuSO₄), Zn is oxidized from 0 to +2 while Cu²⁺ is reduced from +2 to 0.
In cellular respiration, glucose (C₆H₁₂O₆) is oxidized to produce carbon dioxide (CO₂) while oxygen (O₂) is reduced.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Oxidation makes you ‘O’ range, Reduction brings the ‘R’ change.
Stories
Imagine a race where the 'Ox' is losing electrons to the 'Red,' who gains them—this is their moment of transformation together!
Memory Tools
To remember which is which: OIL RIG - Oxidation Is Loss, Reduction Is Gain.
Acronyms
Remember ROS for Redox Oxygen Shift, meaning oxidation reduces and reduction oxidizes.
Flash Cards
Glossary
- Redox Reaction
A chemical reaction involving the transfer of electrons, where one substance is oxidized and another is reduced.
- Oxidation
The process of losing electrons by a molecule, atom, or ion.
- Reduction
The process of gaining electrons by a molecule, atom, or ion.
- Oxidation State
A concept that indicates the degree of oxidation of an atom in a chemical compound.
- Halfreaction
A representation of either the oxidation or reduction part of a redox reaction.
Reference links
Supplementary resources to enhance your learning experience.
