Bond Polarity, Molecular Polarity, and Physical Properties
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Bond Polarity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

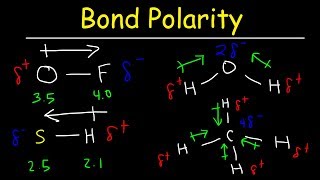
Today, we're going to talk about bond polarity. When two atoms form a bond, the difference in their electronegativity can lead to uneven sharing of electrons. This leads to a bond being polarized.

Can you explain what electronegativity means?

Absolutely! Electronegativity is a measure of an atom's ability to attract electrons in a bond. For example, in a bond between hydrogen and fluorine, fluorine is more electronegative, pulling the shared electrons closer to itself.

So, does that make the bond polar?

Exactly! This uneven sharing creates a dipole moment, where one end of the bond is slightly negative and the other is slightly positive.

Can we visualize this dipole moment?

Yes, we can draw an arrow pointing towards the more electronegative atom, indicating the direction of the dipole. Remember: more electronegative means a stronger pull on the electrons.

How does bond polarity affect molecular polarity?

Great question! If a molecule has polar bonds that do not symmetrically cancel each other out, the molecule itself will have a net dipole moment, making it polar.

In summary, bond polarity is crucial for understanding molecular polarity, which influences physical properties like boiling points.
Exploring Molecular Polarity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s explore how molecular geometry affects polarity. Essentially, the shape of the molecule determines whether the dipole moments cancel out.

What shape would lead to a nonpolar molecule?

A symmetrical shape, like tetrahedral in carbon tetrachloride, can cancel out the bond dipoles. As a result, CCl₄ is a nonpolar molecule despite having polar bonds.

What about water? I thought it has polar bonds!

Exactly! Water is bent, which means the dipole moments do not cancel, leading to a polar molecule with unique properties.

So, what does this mean for physical properties?

Polar molecules tend to have higher boiling points and are usually soluble in polar solvents due to stronger intermolecular forces like hydrogen bonding.

And nonpolar molecules?

They primarily rely on London dispersion forces, which are weaker, resulting in lower boiling points and solubility in nonpolar solvents.

In summary, the geometry of molecules plays an essential role in determining their polarity, which in turn affects their physical properties.
Intermolecular Forces and Physical Properties
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s connect molecular polarity with intermolecular forces! Polar molecules engage in dipole-dipole interactions and hydrogen bonding.

What do those interactions mean for things like boiling point?

Good question! Molecules with strong intermolecular forces require more energy to separate, resulting in higher boiling and melting points.

Can you give an example?

Sure! Compare n-butane, which has a boiling point of –0.5 °C due to London dispersion forces, with 1-butanol, which boils at 117.7 °C because of hydrogen bonding.

Ah, so solubility is also affected!

Exactly! Polar molecules tend to dissolve in polar solvents while nonpolar dissolve in nonpolar solvents—this is 'like dissolves like.'

But what about molecules with larger size?

Larger molecules can be more polarizable, which increases their dispersion forces, further affecting physical properties.

In summary, molecular polarity influences intermolecular forces, leading to significant differences in physical properties.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section explains the difference between polar and nonpolar molecules, the role of dipole moments in molecular interactions, and the effects on physical properties like boiling points and solubility. It emphasizes how molecular shape and intermolecular forces govern these properties.
Detailed
Bond Polarity, Molecular Polarity, and Physical Properties
In this section, we explore the critical aspects of bond polarity and molecular polarity and their relationship with various physical properties of substances. Bond polarity is determined by the difference in electronegativity between atoms in a bond, leading to a partial positive or negative charge. A polar molecule, which has a net dipole moment, engages in stronger intermolecular forces such as dipole-dipole interactions and hydrogen bonding, generally resulting in higher boiling points and melting points. In contrast, nonpolar molecules primarily exhibit London dispersion forces, leading to lower boiling points and solubility in nonpolar solvents.
Trends are illustrated through comparisons of homologous series like alkanes and alcohols, showcasing that isomeric compounds can exhibit vastly different physical behaviors due to their molecular structure and polarity. Furthermore, polarizability is discussed with regard to dispersion forces, where larger electron clouds contribute to stronger intermolecular attractions. Overall, understanding how polarity and intermolecular forces affect the physical properties of substances is crucial in chemistry.
Youtube Videos


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Relationship between Molecular Polarity and Intermolecular Forces
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Polar vs. nonpolar molecules:
○ Polar molecules (with net dipole moment) engage in dipole–dipole and, if applicable, hydrogen bonding. This leads to higher boiling points, melting points, and solubilities in polar solvents.
○ Nonpolar molecules rely primarily on London dispersion forces; they tend to have lower boiling points and are soluble in nonpolar solvents.
● Examples of trends in homologous series (alkanes vs. alcohols):
○ Compare n-butane (C₄H₁₀, nonpolar) and 1-butanol (C₄H₉OH, polar).
■ n-Butane boiling point: –0.5 °C (dispersion only).
■ 1-Butanol boiling point: 117.7 °C (hydrogen bonding).
Detailed Explanation
This chunk explains the relationship between molecular polarity and the types of intermolecular forces that molecules experience. Polar molecules, which have a net dipole moment, can form stronger interactions like dipole-dipole or hydrogen bonds. These interactions cause polar substances to exhibit higher boiling points and melting points compared to nonpolar substances, which primarily rely on weaker London dispersion forces. For instance, n-butane (a nonpolar molecule) has a low boiling point of -0.5 °C because it experiences only weak dispersion forces. In comparison, 1-butanol, which is polar and can form hydrogen bonds, has a boiling point of 117.7 °C due to these stronger interactions.
Examples & Analogies
Imagine polar molecules as friends in a close-knit group. They hold onto each other tightly (forming strong interactions) and create significant warmth (high boiling and melting points). Nonpolar molecules, on the other hand, are like distant acquaintances who only shake hands occasionally (weak London dispersion forces). This leads to them being less stable and ‘cooler’ (lower boiling points) compared to their tightly bonded friends.
Dipole Moment and Solubility
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Dipole moment and solubility:
○ A molecule with a larger dipole moment often dissolves better in polar solvents. For example, acetone (dipole ~2.88 D) is miscible with water, whereas diethyl ether (dipole ~1.15 D) is only slightly soluble (~7 g per 100 g water).
Detailed Explanation
This chunk highlights how the dipole moment of a molecule affects its solubility in polar solvents. A higher dipole moment indicates a stronger polar character, which leads to better interactions with polar solvents like water. For example, acetone, which possesses a significant dipole moment of approximately 2.88 D, dissolves well in water, indicating compatibility between the polar solvent and the polar solute. On the other hand, diethyl ether, which has a lower dipole moment of around 1.15 D, has limited solubility in water, illustrating that weaker polar characteristics do not favor dissolution in polar environments.
Examples & Analogies
Think of polar solvents like water as a group of individuals who prefer to socialize with friends who have similar interests (polar traits). Acetone is like a new friend who fits in perfectly with this group and thus can easily mingle and dissolve. In contrast, diethyl ether is less compatible; it finds a smaller circle within the group and struggles to connect, leading to its low solubility.
Polarizability and Dispersion Forces
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Polarizability (α): The ease with which an electron cloud can be distorted.
○ Larger atomic or molecular size → more polarizable → stronger dispersion forces.
○ Examples:
■ Iodine (I₂) has a larger, more polarizable electron cloud than fluorine (F₂). Consequently, I₂ is a solid at room temperature (melting point 113.7 °C, boiling point 184.3 °C) while F₂ is a gas (boiling point –188.1 °C).
■ Among hydrocarbons, branched isomers have less surface contact and thus lower boiling points than their straight-chain counterparts. Compare n-hexane (boiling point 68.7 °C) vs. 2,2-dimethylbutane (boiling point 49.7 °C).
Detailed Explanation
This chunk discusses polarizability, which defines how easily an electron cloud can be distorted by external forces. Larger atoms or molecules have more polarizable electron clouds, leading to stronger dispersion forces. For instance, iodine (I₂), due to its larger size compared to fluorine (F₂), exhibits more polarizability and thus exists as a solid at room temperature. In hydrocarbons, the structure also influences boiling points; for example, n-hexane, which is a straight-chain hydrocarbon, has a higher boiling point (68.7 °C) compared to its branched isomer, 2,2-dimethylbutane (49.7 °C), because the straight-chain structure offers more surface contact for stronger dispersion forces.
Examples & Analogies
Imagine an inflatable balloon filled with air. The larger the balloon (like iodine's atomic size), the easier it is to squeeze and distort (increase polarizability). In contrast, a small balloon (like fluorine) is less affected by squeezing forces. Similarly, think of n-hexane as a long, straight parade float with more interactions with its surroundings, whereas 2,2-dimethylbutane is like a pruned float that can't interact as readily, resulting in lower boiling points.
Key Concepts
-
Bond Polarity: The distribution of charge in a bond based on electronegativity differences.
-
Molecular Polarity: The overall dipole moment of a molecule determined by the shape and polarity of its bonds.
-
Intermolecular Forces: The forces that exist between molecules that affect physical properties.
-
Polar vs Nonpolar Molecules: Polar molecules have net dipole moments; nonpolar molecules do not.
-
Influence on Physical Properties: Polarity affects boiling points, melting points, and solubility.
Examples & Applications
Water (H₂O) is polar with a bent shape causing a net dipole moment.
Carbon tetrachloride (CCl₄) is nonpolar due to its symmetrical tetrahedral geometry despite having polar C-Cl bonds.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
A polar bond, a dipole drawn, dipole's strength comes at dawn. With forces strong, they won't be wrong, higher boiling point all along.
Stories
Once in a land of molecules, there lived a polar water molecule. Always looking for friends, it found many other polar friends due to its strong dipole moment, while its nonpolar neighbors like butane struggled to find a partner to dance with!
Memory Tools
Remember 'Like Dissolves Like' as a guide to understand solubility: Polar with polar, nonpolar with nonpolar.
Acronyms
H2O
Hydrophilic (water-loving)
Polar (Net dipole)
Soluble (in water).
Flash Cards
Glossary
- Bond Polarity
The unequal distribution of electron density between two bonded atoms due to differences in electronegativity.
- Molecular Polarity
A measure of the net dipole moment of a molecule, determined by both bond polarity and molecular geometry.
- Dipole Moment
A vector that represents the separation of positive and negative charges within a molecule.
- Polar Molecule
A molecule that has a net dipole moment and asymmetrically distributed charges.
- Nonpolar Molecule
A molecule that has no net dipole moment, often due to symmetric charge distribution.
- Intermolecular Forces
Forces of attraction between molecules that determine physical properties such as boiling point and solubility.
- Polarizability
The ease with which the electron cloud of an atom or molecule can be distorted, affecting its intermolecular forces.
Reference links
Supplementary resources to enhance your learning experience.
