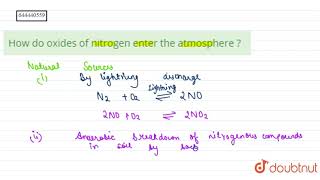
Nitrogen Oxides (NO, NO₂)
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Nitrogen Oxides
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we will learn about nitrogen oxides, specifically nitric oxide (NO) and nitrogen dioxide (NO₂). Can anyone share what they know about these gases?

I've heard they're linked to pollution. Is that true?

Absolutely! Nitrogen oxides are key pollutants primarily produced by vehicle emissions and high-temperature combustion processes. They play a significant role in atmospheric pollution. What do you think happens when these gases are released into the environment?

Maybe they contribute to smog or other environmental issues?

Yes! They can cause acid rain and respiratory issues in humans, among other harmful effects. Remember, the acronym 'NOx' refers to both nitric oxide and nitrogen dioxide.
Sources of Nitrogen Oxides
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's explore where nitrogen oxides come from. Can anyone think of sources of these gases?

I think cars are a major source because of the exhaust.

Exactly! Vehicle exhaust is a primary source. High-temperature combustion in factories and power plants also releases significant amounts of NO and NO₂. Why do you think high temperatures are necessary for their formation?

Because it helps break down nitrogen in the air?

Spot on! The heat enables nitrogen and oxygen molecules in the air to react. Great reasoning!
Effects of Nitrogen Oxides
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we know the sources, let's talk about their effects. How might nitrogen oxides affect our health?

They can cause breathing problems, right?

Correct! Exposure can lead to respiratory issues and worsen conditions like asthma. Can anyone mention the environmental effects?

I think they can cause acid rain, too.

Exactly! Nitrogen oxides react with water in the atmosphere forming nitric acid, which contributes to acid rain. This is harmful to plants, aquatic ecosystems, and buildings. Remember that.
Connecting the Dots
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To conclude, why do you think it's important to control nitrogen oxide emissions?

To reduce health problems and environmental damage!

Absolutely! Reducing these emissions is crucial for improving air quality and protecting our health and the environment. Let's remember that NOx stands for both nitric oxide and nitrogen dioxide, which are central to our discussions today.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Nitrogen oxides, primarily constituted by nitric oxide (NO) and nitrogen dioxide (NO₂), are significant pollutants derived from vehicle emissions and high-temperature combustion. They contribute to environmental issues such as acid rain and respiratory irritations.
Detailed
Nitrogen Oxides (NO, NO₂)
Nitrogen oxides (NO and NO₂) are vital pollutants that play a detrimental role in atmospheric pollution. They are primarily generated from vehicle exhaust systems and high-temperature combustion processes, such as those occurring in power plants and engines.
Sources:
- Vehicle Exhaust: Cars, trucks, and buses emit nitrogen oxides as a result of fuel combustion.
- High-Temperature Combustion: Burning fossil fuels in power plants and industrial processes also releases these gases.
Effects:
- Acid Rain: Nitrogen oxides react with water vapor and other substances in the atmosphere to form nitric acid, leading to acid rain, which can harm vegetation, aquatic life, and soil.
- Respiratory Problems: Exposure to nitrogen oxides can irritate the airways, leading to respiratory conditions and exacerbating asthma symptoms.
Understanding the significance of nitrogen oxides in atmospheric chemistry is crucial for developing strategies to combat air pollution and protect public health.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Source of Nitrogen Oxides
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Source: Vehicle exhausts, combustion at high temperature
Detailed Explanation
Nitrogen oxides (NO and NO₂) are primarily produced from two sources: vehicle exhaust gases and high-temperature combustion processes. Vehicles burn fuel, which generates heat, and during this process, nitrogen in the air reacts with oxygen to form NO and NO₂. Additionally, industrial processes that involve combustion at high temperatures, such as power plants, also release these gases into the atmosphere.
Examples & Analogies
Imagine cooking food at a high temperature; just like how the heat cooks the food and creates smoke in your kitchen, high temperatures from vehicles and factories produce nitrogen oxides as a byproduct of burning fuel.
Effects of Nitrogen Oxides
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Effect: Acid rain, respiratory irritation
Detailed Explanation
Nitrogen oxides have several harmful effects on the environment and human health. When NO and NO₂ are released into the atmosphere, they can react with moisture and other chemicals to form acids, which leads to acid rain. This acid rain can significantly harm ecosystems, buildings, and water sources. Additionally, inhaling these gases can cause respiratory problems in people, leading to issues such as asthma and chronic bronchitis.
Examples & Analogies
Think of nitrogen oxides as uninvited guests at a party. They might cause trouble (like acid rain) that spoils the fun (healthy environments and people). Just as you'd want to avoid inviting troublesome guests, we need to reduce the emissions that let nitrogen oxides into our atmosphere.
Key Concepts
-
Nitrogen Oxides: Pollutants including NO and NO₂ that arise from combustion processes.
-
Sources: Vehicle emissions and industrial combustion are primary sources of nitrogen oxides.
-
Effects: Contributes to respiratory issues and acid rain formation.
Examples & Applications
Example 1: A car engine emits NO as fuel is burned, contributing to urban smog.
Example 2: A factory utilizing coal releases NO₂ as a byproduct of fossil fuel combustion.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Nitrogen oxides in the sky, make the air very high.
Stories
Once, in a bustling city, cars raced by, creating a thick fog. This fog contained nitrogen oxides, which caused the rain to fall with a sting, hurting trees and making it hard for people to breathe. But then, the city learned to control those gases, and the fog faded away, replacing it with clear skies!
Memory Tools
N.O. = Not Okay! Whenever you see nitrogen oxides in the air, remember they're causing trouble!
Acronyms
NOx = Nitric Oxide and Nitrogen Dioxide – two main gases contributing to air pollution.
Flash Cards
Glossary
- Nitric Oxide (NO)
A nitrogen oxide that is significant in atmospheric chemistry and contributes to air pollution.
- Nitrogen Dioxide (NO₂)
A reddish-brown gas that is a key pollutant formed from combustion processes and leads to respiratory issues.
- Acid Rain
Precipitation with acidic components that results from nitrogen oxides and sulfur dioxide reacting with water.
Reference links
Supplementary resources to enhance your learning experience.
