Reversed Carnot Cycle
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to the Reversed Carnot Cycle
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we start discussing the Reversed Carnot Cycle, which is central to understanding refrigeration systems. What do you think is the importance of having an ideal cycle?

I think it sets a standard for efficiency.

Yes, but how does it actually work?

Great questions! The Reversed Carnot Cycle consists of four processes. First, we have isothermal heat absorption at a low temperature, which is essential for the cycle to function efficiently.

What does isothermal mean?

Isothermal means that the temperature remains constant during heat absorption. This efficiency is part of why these cycles are idealized.

So it can absorb heat but doesn't change temperature while doing it?

Exactly! Let’s keep building on this concept by exploring the next process — isentropic compression.

To recap, the Reversed Carnot Cycle sets a theoretical benchmark for refrigeration efficiency through its four distinct processes.
Understanding COP
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

The Coefficient of Performance, or COP, plays a crucial role in refrigeration systems. Can anyone tell me what COP signifies?

Isn’t it a measure of efficiency?

Correct! It represents the ratio of useful refrigeration to the work input. The formula for COP in refrigeration is given by $$ COP_{ref} = \frac{T_L}{T_H - T_L} $$. Who can explain why we use T_L and T_H?

T_L is the low temperature, and T_H is the high temperature. They define the limits within which the cycle operates.

Exactly! Remember, the higher the COP, the better the performance of our refrigeration system. Now, what are some limitations of the Reversed Carnot Cycle?

It relies on isothermal processes that are hard to achieve in real life.

Exactly right! These processes make it ideal but impractical. To summarize, COP is a critical factor that defines the theoretical efficiency of our system.
Limitations and Applications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's dive into some limitations and their implications. Why is the Reversed Carnot Cycle described as purely theoretical?

Because it requires isothermal conditions that can't be achieved with gases on a large scale.

Exactly! And despite these limitations, this cycle is used as a benchmark. Can anyone explain what that means?

It helps us compare other real-world refrigeration cycles against an ideal standard.

Great insight! So to summarize today, the Reversed Carnot Cycle, while not practical, serves a significant role in understanding refrigeration efficiency.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section discusses the Reversed Carnot Cycle, its principles, key features, limitations, and its role as a theoretical benchmark for refrigeration systems. Although it offers the highest Coefficient of Performance (COP), it is impractical for actual use due to theoretical constraints.
Detailed
Reversed Carnot Cycle
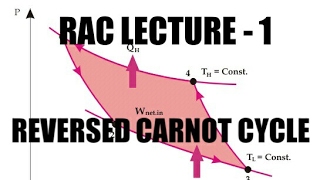
The Reversed Carnot Cycle is an ideal refrigeration cycle that is constructed to operate with maximum theoretical efficiency. It uniquely utilizes air as the working fluid and consists of four reversible processes:
- Isothermal Heat Absorption (at low temperature, T_L) - This process involves absorbing heat from a cold reservoir at low temperature, keeping the temperature constant during absorption.
- Isentropic Compression - Following heat absorption, the compressed air is heated and pressurized without loss of entropy.
- Isothermal Heat Rejection (at high temperature, T_H) - Warm air releases heat to a hot reservoir while maintaining a constant temperature.
- Isentropic Expansion - The air expands, decreasing both temperature and pressure.
Key Features
- Coefficient of Performance (COP): Represents the highest possible value achievable under specified temperature limits, specifically for refrigeration it’s defined as
$$ COP_{ref} = \frac{T_L}{T_H - T_L} $$ - Limitations: Although theoretically highly efficient, it requires isothermal processes which are not practical for large scale applications. Additionally, the required equipment would be large and operate slowly.
Applications
The Reversed Carnot Cycle serves principally as a benchmark for evaluating the performance of practical refrigeration cycles, but it is not implemented in real-world air refrigeration systems due to the outlined practical challenges.
Youtube Videos



Audio Book
Dive deep into the subject with an immersive audiobook experience.
Principle of the Reversed Carnot Cycle
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The reversed Carnot cycle is an ideal refrigeration cycle conceived for maximum theoretical efficiency. It uses air as the working fluid and consists of four reversible processes:
- Isothermal Heat Absorption (at low temperature, TL)
- Isentropic Compression
- Isothermal Heat Rejection (at high temperature, TH)
- Isentropic Expansion
Detailed Explanation
The reversed Carnot cycle is a theoretical model used to describe the process of refrigeration. Its goal is to achieve maximum efficiency in cooling. The cycle includes four key processes:
1. Isothermal Heat Absorption: The system absorbs heat from a low-temperature source without changing the temperature, providing the initial cooling effect.
2. Isentropic Compression: The working fluid (air) is compressed, increasing its pressure and temperature without losing energy.
3. Isothermal Heat Rejection: The system releases heat to a high-temperature sink while maintaining a constant temperature.
4. Isentropic Expansion: The air expands, dropping in pressure and allowing for further cooling.
Each of these processes plays a critical role in the cycle's efficiency.
Examples & Analogies
Think of the reversed Carnot cycle like a highly efficient refrigerator. Imagine putting your groceries in the fridge on a hot day. The fridge takes in heat from inside (like the warm groceries), compresses it to handle the heat better, pushes the heat outside, and then creates a chilly space for your food to stay cool. However, like a superhero who needs perfect conditions to work, this cycle operates under idealized conditions that aren't always practical in real life.
Key Features of the Reversed Carnot Cycle
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
COP (Coefficient of Performance): Highest possible for given temperature limits.
COP for refrigeration: $ \frac{T_L}{T_H - T_L} $
Limitation: Purely theoretical; requires isothermal processes (not practical with gases at large scales).
Large, impractical equipment sizes and slow operation.
Detailed Explanation
The efficiency of the reversed Carnot cycle is measured by the Coefficient of Performance (COP), which is defined as the ratio of the temperature of the cold source (TL) to the difference between the high temperature (TH) and the low temperature (TL). This gives the reversed Carnot cycle the highest theoretical COP compared to other refrigeration cycles.
However, the cycle is primarily theoretical. In practice, achieving the perfectly isothermal processes required is very challenging, particularly for gases over large scales. Also, the equipment needed for this cycle tends to be large and cumbersome, making it impractical for everyday use. The slow operation of these systems further limits their applicability.
Examples & Analogies
Imagine trying to set up a flawless air conditioning system in your home using only theoretical concepts. While it sounds perfect on paper (like our ideal unit), as soon as you try to build it, you find out you need a giant unit that takes forever to cool down the room. In the real world, we need to compromise for practicality, leading to designs that are efficient but not as perfect as the Carnot cycle suggests.
Limitations of the Reversed Carnot Cycle
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Limitation: Purely theoretical; requires isothermal processes (not practical with gases at large scales).
Large, impractical equipment sizes and slow operation.
Detailed Explanation
The limitations of the reversed Carnot cycle stem from its reliance on idealized processes. For it to function as intended, all heat transfer must occur isothermally, which is nearly impossible with gases in real-world scenarios. Additionally, the equipment required for this cycle tends to be too large for practical applications and operates slowly. Therefore, while it serves as an important theoretical benchmark, it cannot be effectively implemented in practical refrigeration systems.
Examples & Analogies
Consider a recipe for a perfect cake that requires precise conditions and ingredients. If your kitchen doesn’t have the right tools or environment (like an ideal oven that maintains exact temperatures), you can’t recreate that perfect cake. Similarly, the reversed Carnot cycle outlines a 'perfect' refrigeration system, but the practical hurdles mean we often settle for less than the ideal scenario.
Applications of the Reversed Carnot Cycle
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Serves as a benchmark for comparison with practical cycles.
Not used in actual air refrigeration systems due to practical implementation challenges.
Detailed Explanation
One of the main applications of the reversed Carnot cycle lies in its function as a theoretical benchmark. It provides a standard against which other, more practical refrigeration cycles can be compared. However, given the practical difficulties tied to its idealized processes, it is not used in real-life air refrigeration systems, where alternative cycles are preferred.
Examples & Analogies
Think of the reversed Carnot cycle as a perfect score in an exam. While it sets a high standard for students, not every student can achieve that score. It serves as a measure of potential, helping students identify areas for improvement, just as the Carnot cycle helps engineers assess and improve real refrigeration technologies.
Key Concepts
-
Reversed Carnot Cycle: An idealized refrigeration process that maximizes efficiency.
-
Coefficient of Performance (COP): A critical measure of refrigeration effectiveness, represented by a ratio between useful energy output and work input.
-
Isothermal and Isentropic Processes: Crucial processes in thermodynamics that respectively involve constant temperature and entropy.
Examples & Applications
The Reversed Carnot Cycle serves as a theoretical model for designing more practical refrigeration systems.
When engineers calculate the COP for real systems, they often do so relative to the idealized values predicted by the Reversed Carnot Cycle.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Carnot’s cycle runs both ways, cold to hot, it displays. Efficient flow, isothermal show, in refrigeration, it's the star of the show!
Stories
Imagine a magician at a carnival. He waves his wand (the compressor) while the cold air (low temperature) magically absorbs heat from the crowd, then he releases warmer air to the sky (higher temperature) before vanishing into thin air (isothermal processes).
Memory Tools
I-ICE: Isothermal Heat absorption, Isentropic Compression, Isothermal Heat rejection, Isentropic Expansion.
Acronyms
COLD
Carnot's Optimal Loop for Dynamics - representing the essence of the reversed cycle.
Flash Cards
Glossary
- Reversed Carnot Cycle
An ideal refrigeration cycle designed for maximum theoretical efficiency using air as a working fluid.
- Coefficient of Performance (COP)
A measure of the efficiency of refrigeration systems, defined as the ratio of useful refrigeration to work input.
- Isothermal Process
A thermodynamic process in which the temperature remains constant.
- Isentropic Process
A thermodynamic process that is both adiabatic and reversible, with no entropy change.
Reference links
Supplementary resources to enhance your learning experience.
