Ideal Vapor Compression Refrigeration (VCR) Cycle
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Ideal VCR Cycle
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are going to discuss the ideal vapor compression refrigeration cycle which consists of four main processes. Can anyone tell me the first process?

Is it the compression of the refrigerant?

Exactly! This process is called isentropic compression. The refrigerant vapor is compressed, raising its pressure and temperature. Can anyone remember what happens next?

I think it goes through the condenser where it releases heat.

Correct! This is called isobaric condensation. Here, the vapor releases heat and turns into a high-pressure liquid. Let's remember this as 'Cool Down in Condensation'. Now, what follows after condensation?

The refrigerant goes through an expansion valve.

Yes, this is the isenthalpic expansion process! The refrigerant reduces its pressure and temperature. Finally, what happens in the last step?

The low-pressure liquid absorbs heat and turns back into vapor.

That's right! This is isobaric evaporation. Great job, everyone! Remember: Compression, Condensation, Expansion, and Evaporation. Can anyone summarize what we learned?

We learned about the four processes of the ideal VCR cycle!
Understanding the Coefficient of Performance (COP)
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's discuss the Coefficient of Performance, or COP, which measures the efficiency of the refrigeration cycle. What would you expect the COP to reflect in our ideal cycle?

It should indicate how well the cycle performs, right?

Exactly! In the ideal VCR cycle, the COP is theoretically very high because we assume no losses—it's a perfect scenario. But what about real cycles? Why do you think they have lower COP?

Because of inefficiencies like heat loss and pressure drops?

Correct! Real systems face irreversibilities that affect their performance. Remember: 'Higher COP, Ideal Cycle; Lower COP, Real World.' Now, who can recall what COP means in simple terms?

It's the ratio of useful heating or cooling provided to the work required.

Well done! Understanding COP is essential for analyzing the efficiency of refrigeration systems.
Limitations of the Ideal VCR Cycle
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

We've discussed what the ideal VCR cycle looks like, but let's talk about the limitations. Can anyone identify why the ideal cycle might not be practical?

It assumes perfect performance with no losses.

Right! The ideal cycle neglects real-world inefficiencies such as pressure drops and non-isentropic processes. Why is this important?

It makes it hard to apply to actual refrigeration systems?

Exactly! It's crucial to use this ideal model for comparison purposes, not as a blueprint for what to expect in reality.
Review of VCR Cycle Processes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's do a quick review! Can anyone tell me the sequence of the ideal VCR cycle processes again?

It starts with compression, then condensation, followed by expansion, and finally evaporation!

Great job! Can someone explain what happens during the condensation process?

The vapor releases heat and condenses into a liquid.

Exactly! This process is crucial to lowering the refrigerant's temperature. Well done, everyone! Remember these key processes for understanding refrigeration systems.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
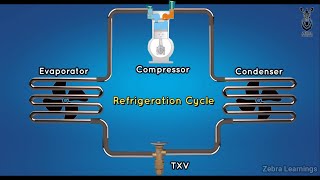
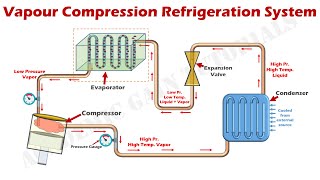
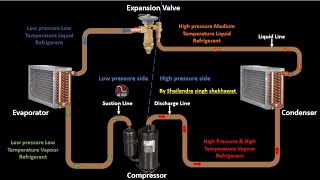
The ideal VCR cycle is structured around four processes: compression, condensation, expansion, and evaporation. It serves as a theoretical model that highlights the idealized performance of refrigeration systems, providing benchmarks against which real-world systems can be compared.
Detailed
Detailed Summary of the Ideal Vapor Compression Refrigeration (VCR) Cycle
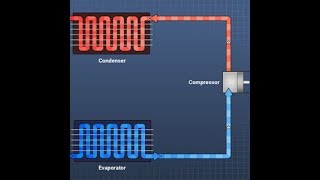
The ideal vapor compression refrigeration (VCR) cycle is a foundational concept in thermodynamics that illustrates how mechanical energy is converted into heat transfer for cooling purposes. The cycle is comprised of four essential processes:
- Isentropic Compression: In this initial stage, the refrigerant vapor is compressed by the compressor, resulting in an increase in both pressure and temperature.
- Isobaric Condensation: The high-pressure, high-temperature vapor then transitions to the condenser where it releases heat to the environment, thereby condensing into a high-pressure liquid.
- Isenthalpic Expansion: The liquid refrigerant experiences a reduction in pressure as it flows through an expansion valve, which occurs in an essentially constant-enthalpy process, and its temperature decreases.
- Isobaric Evaporation: Finally, the low-pressure liquid-vapor mixture absorbs heat in the evaporator, converting into low-pressure vapor and completing the cycle.
While the ideal VCR cycle illustrates perfect component operation with no losses or inefficiencies, it also serves as a benchmark against which real, practical VCR systems can be measured. Understanding this cycle is essential for analyzing actual performances, which include various inefficiencies such as pressure drops and non-isentropic processes, ultimately impacting the Coefficient of Performance (COP).
Youtube Videos
Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to the Ideal VCR Cycle
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
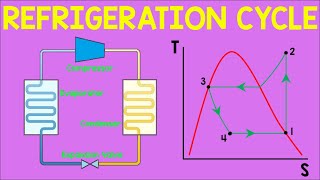
The ideal VCR cycle is a thermodynamic model that outlines how mechanical energy is used to transfer heat from a low-temperature region to a high-temperature region using a circulating refrigerant. The cycle consists of four basic processes, represented on P-h and T-s diagrams:
Detailed Explanation
The ideal Vapor Compression Refrigeration (VCR) cycle serves as a theoretical framework to understand how refrigeration systems function. This model demonstrates how a refrigerant absorbs heat from a colder space and releases it into a warmer area, facilitated by mechanical energy provided by a compressor. It is essential that the refrigerant circulates through four distinct processes to achieve this heat transfer effectively.
Examples & Analogies
Imagine a sponge soaking up water from a small puddle (the low-temperature area) and then squeezing out that water onto a hot pavement (the high-temperature area). The sponge represents the refrigerant, and the action of squeezing is akin to the work done by the compressor in the refrigeration cycle.
Four Basic Processes of the Cycle
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Isentropic Compression: Refrigerant vapor is compressed by a compressor, raising its pressure and temperature.
- Isobaric Condensation: The high-pressure, high-temperature vapor flows through a condenser, releasing heat to the surroundings and condensing to a high-pressure liquid.
- Isenthalpic Expansion: The liquid refrigerant passes through an expansion valve (throttle), reducing its pressure and temperature in an essentially constant-enthalpy process.
- Isobaric Evaporation: Low-pressure liquid-vapor mixture absorbs heat in the evaporator, turning into low-pressure vapor and completing the cycle.
Detailed Explanation
The cycle consists of four sequential processes, each crucial for efficient refrigeration:
1. Isentropic Compression: The refrigerant enters the compressor as a vapor, where it is compressed, resulting in increased pressure and temperature, transforming it into a high-energy state.
2. Isobaric Condensation: The high-energy refrigerant vapor is then cooled in the condenser, releasing heat to the environment, which causes the refrigerant to condense into a liquid at high pressure.
3. Isenthalpic Expansion: The high-pressure liquid refrigerant passes through an expansion valve, where it experiences a pressure drop and a reduction in temperature while maintaining enthalpy.
4. Isobaric Evaporation: Finally, in the evaporator, the low-pressure liquid absorbs heat from the surrounding environment and evaporates back into vapor, completing the cycle.
Examples & Analogies
Think of the cycle like a bicycle pump:
1. When you compress the pump handle (isentropic compression), the air inside gets compressed and heated up.
2. When you release the handle (isobaric condensation), the accumulated air pressure tries to push the air out, allowing hot air to exit.
3. Opening a valve allows the air pressure to drop (isenthalpic expansion), cooling the air down.
4. Finally, when you pull the handle again (isobaric evaporation), the surrounding air is drawn in, completing the pumping action.
Key Features and Limitations of the Ideal VCR Cycle
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Key Features: The cycle is reversible; no pressure drops, no losses; all components operate ideally.
- Limitations: Neglects real-world inefficiencies (e.g., pressure drops, non-isentropic compression, heat losses). Assumes perfect component operation (no subcooling, superheating, or irreversibilities). Not attainable in practice but serves as a reference for performance comparison.
Detailed Explanation
The ideal VCR cycle is characterized by features that represent its perfect operation. It is portrayed as reversible, meaning it can theoretically run in either direction without losses, and all components are assumed to function ideally without any inefficiencies. However, this is an unrealistic representation. In practice, various inefficiencies and losses occur due to imperfect mechanical operations, pressure drops, and heat loss, making the ideal VCR cycle unattainable in real-world applications. Nevertheless, it provides a valuable comparison point for evaluating actual VCR systems.
Examples & Analogies
Consider a perfect machine that can operate without any friction (ideal VCR). In reality, all machines experience wear and lose energy, much like how a perfectly smooth road exists only in theory. Every car on a real road encounters bumps and friction, increasing fuel consumption. Similarly, while the ideal cycle serves as a benchmark, no existing refrigeration system can achieve this perfection.
Key Concepts
-
Isentropic Compression: The process where refrigerant vapor is compressed without heat loss, increasing its pressure and temperature.
-
Isobaric Condensation: The phase change from vapor to liquid where heat is expelled at constant pressure.
-
Isenthalpic Expansion: The reduction of refrigerant pressure while maintaining its total enthalpy.
-
Isobaric Evaporation: The conversion of liquid refrigerant to vapor by heat absorption at constant pressure.
-
Coefficient of Performance (COP): A key measure indicating the efficiency of a refrigeration cycle.
Examples & Applications
An example of the ideal VCR cycle is used in many household refrigerators, where the refrigerant follows the four processes to keep the interior cool.
An air conditioning system also follows the VCR cycle, whereby the refrigerant absorbs heat from the indoor air and releases it outside.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
From compression to condensation, through expansion, we get our evaporation, cooling is the goal of our refrigeration!
Stories
Imagine a refrigerant traveling on a journey: first it gets pumped up high in pressure, then it cools down as it releases heat, next it takes a chill pill dropping pressure before finally enjoying warmth as it absorbs heat from its surroundings before starting again.
Memory Tools
Remember 'C-C-E-E' for Compression, Condensation, Expansion, and Evaporation.
Acronyms
ICE for ideal VCR Cycle
for Isentropic
for Condensation
and E for Evaporation processes.
Flash Cards
Glossary
- Vapor Compression Refrigeration (VCR) Cycle
A thermodynamic cycle that uses mechanical energy to transfer heat from a low-temperature region to a higher one using a refrigerant.
- Isentropic Compression
A compression process that occurs without a change in entropy, typically idealized in thermodynamic cycles.
- Isobaric Condensation
A process where a vapor releases heat to become a liquid at constant pressure.
- Isenthalpic Expansion
An expansion process where the enthalpy remains constant as pressure decreases, typically occurring in expansion valves.
- Isobaric Evaporation
The phase change process where a liquid absorbs heat and turns into vapor at constant pressure.
- Coefficient of Performance (COP)
A measure of the efficiency of a refrigeration cycle, defined as the ratio of useful cooling provided to the work input.
Reference links
Supplementary resources to enhance your learning experience.
