Introduction to Solid, Liquid and Gaseous Fuels
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
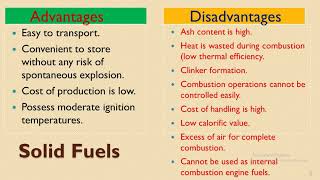
Solid Fuels
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will begin with solid fuels. Can anyone name some examples of solid fuels?

Coal and wood.

What about coke?

Excellent! Coal, wood, and coke are indeed solid fuels. They come from organic materials and vary in terms of their calorific values and environmental effects. For example, wood has a lower calorific value than coal.

Why is calorific value important?

Good question! The calorific value tells us how much energy we can get from a certain amount of fuel. It's crucial for determining how much fuel we need for specific energy requirements. We can remember calorific value with the acronym CV—C for the calorie and V for value!

What else should we know about these fuels?

Besides calorific value, we also need to consider moisture and ash content, as well as volatility and ignition temperature. These factors influence efficiency and emissions. Let's summarize with these key points: Solid fuels are diverse, with varying energy outputs and environmental impacts!
Liquid Fuels
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let's dive into liquid fuels. Can anyone list some types of liquid fuels?

Petrol and diesel!

What about kerosene?

Exactly, petrol, diesel, and kerosene are key liquid fuels. Liquid fuels tend to have high energy densities. This means they can release a lot of energy in a small volume. Remember the mnemonic 'P-D-K' for Petrol, Diesel, Kerosene!

Are there any downsides to using liquid fuels?

Yes, while they are efficient, they also come with environmental concerns, such as air pollution and greenhouse gas emissions. Understanding the balance between efficiency and sustainability is essential. Let’s recap: Liquid fuels offer high energy density but warrant careful environmental considerations.
Gaseous Fuels
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let's talk about gaseous fuels. What are some of the gaseous fuels we're familiar with?

Natural gas and biogas!

And LPG, right?

Correct! Natural gas, LPG, and biogas are examples of gaseous fuels that are known for their clean-burning properties. This means they produce fewer pollutants compared to solid and liquid fuels. You can remember this with the phrase 'Clean Gas Wins'!

How do we measure the efficiency of these fuels?

We measure efficiency through calorific value and emissions analysis. Gaseous fuels often have a high calorific value combined with lower emissions, making them a favorable choice for future energy solutions. Let's solidify this by summarizing: Gaseous fuels provide a cleaner alternative to traditional fuels, with strong energy outputs.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section covers the three main fuel states: solid fuels (like coal and wood), liquid fuels (such as petrol and diesel), and gaseous fuels (including natural gas and LPG). It also outlines important fuel characteristics, including calorific value, moisture, and volatility.
Detailed
Introduction to Solid, Liquid, and Gaseous Fuels
This section provides an overview of the three primary categories of fuels used in various combustion processes.
Solid Fuels
Solid fuels such as coal, lignite, wood, and coke are derived from organic materials. They present distinct advantages and disadvantages in terms of energy output and environmental impact.
Liquid Fuels
Liquid fuels include petrol, diesel, kerosene, and fuel oils. These fuels are commonly used in transportation and heating due to their high energy density.
Gaseous Fuels
Gaseous fuels consist of natural gas, liquefied petroleum gas (LPG), biogas, and producer gas. They are notable for being clean-burning and efficient.
Fuel Characteristics
Key fuel characteristics discussed include:
- Calorific Value: Divided into Higher Heating Value (HHV) and Lower Heating Value (LHV), it indicates the energy content of the fuel.
- Moisture Content: The water present in the fuel, affecting its energy output.
- Ash Content: Residue left after combustion, influencing environmental emissions.
- Volatility: The tendency of a substance to vaporize, critical for combustion efficiency.
- Ignition Temperature: The minimum temperature needed to start combustion.
Understanding these diverse fuels and their properties is foundational for effective combustion and energy management.
Youtube Videos


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Types of Fuels
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Solid fuels: Coal, lignite, wood, coke
● Liquid fuels: Petrol, diesel, kerosene, fuel oils
● Gaseous fuels: Natural gas, LPG, biogas, producer gas
Detailed Explanation
Fuels are classified into three main types based on their physical state: solid, liquid, and gaseous. Solid fuels include materials like coal, lignite, wood, and coke. Liquid fuels consist of petrol, diesel, kerosene, and various fuel oils. Lastly, gaseous fuels encompass natural gas, liquefied petroleum gas (LPG), biogas, and producer gas. Each type of fuel has its unique properties, uses, and applications in energy production and heating.
Examples & Analogies
Imagine you are camping. You might use wood (solid fuel) to build a campfire, petrol (liquid fuel) for your camping stove, and natural gas (gaseous fuel) at home for cooking. Each fuel type serves specific needs depending on the situation, just as they do in larger energy systems.
Fuel Characteristics
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Fuel characteristics:
● Calorific value (Higher and Lower Heating Value)
● Moisture content, ash content
● Volatility, ignition temperature
Detailed Explanation
The properties of fuels are crucial for understanding their efficiency and suitability for different applications. Key characteristics include:
- Calorific Value: This indicates how much energy is released when a fuel is burned. It can be divided into Higher Heating Value (HHV) and Lower Heating Value (LHV).
- Moisture Content: The amount of water present in the fuel can affect combustion efficiency. Higher moisture content typically reduces energy output.
- Ash Content: This refers to the inorganic residue remaining after combustion, which can influence emissions and waste management.
- Volatility: This property describes how easily a fuel can vaporize, impacting how it is stored and used.
- Ignition Temperature: The minimum temperature at which a fuel will ignite, which affects burner design and operational safety.
Examples & Analogies
Think of fuel characteristics like cooking ingredients. Just as you check the quality and freshness of ingredients (like ensuring your vegetables aren’t wilted or wet), fuel quality is essential for optimal combustion. Using highly volatile and low moisture fuels is like using fresh and flavorful ingredients; it leads to better cooking results (higher efficiency and lower emissions).
Key Concepts
-
Solid Fuels: Fuels in solid state that include coal, lignite, wood, and coke.
-
Liquid Fuels: Fuels in liquid state such as petrol, diesel, kerosene, and fuel oils.
-
Gaseous Fuels: Fuels in gaseous state including natural gas, LPG, biogas, and producer gas.
-
Calorific Value: Measures the energy content of fuels, critical for energy calculations.
-
Moisture Content: Represents the water content in fuels, impacting combustion efficiency.
-
Volatility: Indicates how readily a fuel may vaporize, affecting ignition and combustion.
Examples & Applications
A common example of solid fuel is coal, which is widely used in power generation due to its high calorific value but also associated with significant emissions.
An example of liquid fuel is petrol, which is utilized predominantly in vehicles for its energy density and efficiency.
Natural gas, a gaseous fuel, is preferred in many residential heating applications due to its clean-burning nature.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Solid fuels like coal and wood, burn hot and fast, they do what they should.
Stories
Once upon a time, coal and wood decided to compete in a burning contest. Coal, being solid and dense, won by providing more heat but left a smoky mess, while wood burned peacefully but quickly faded. Later, they met Natural Gas, who burned clean and bright, showing them a better way to power the night.
Memory Tools
For solid fuels remember C-W-C: Coal, Wood, and Coke!
Acronyms
L.F.G. for Liquid Fuels
for Liquid
for Fuel
and G for Gasoline.
Flash Cards
Glossary
- Solid Fuels
Fuels in solid state, such as coal, wood, and coke, used for energy production.
- Liquid Fuels
Fuels in liquid state, including petrol, diesel, and kerosene, commonly used in transportation and heating.
- Gaseous Fuels
Fuels in gaseous state, such as natural gas and LPG, known for clean combustion.
- Calorific Value
The amount of energy produced by the combustion of a fuel, usually measured in MJ/kg.
- Moisture Content
The amount of water present in fuel, affecting combustion efficiency.
- Ash Content
The non-combustible residue left after a fuel is burned.
- Volatility
The tendency of a substance to vaporize, essential for efficient combustion.
- Ignition Temperature
The minimum temperature needed to initiate combustion of a fuel.
Reference links
Supplementary resources to enhance your learning experience.
