Ozone Layer and How it is Getting Depleted
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to the Ozone Layer
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome, class! Today, we will explore the ozone layer. Can anyone tell me what ozone is?

Isn't ozone just a type of oxygen?

Good guess! Ozone consists of three oxygen atoms, making it different from the oxygen we breathe, which is O₂. Does anyone know why the ozone layer is important?

It protects us from the sun's harmful rays!

Exactly! The ozone layer absorbs most of the sun’s ultraviolet (UV) radiation, which can damage our skin. Remember: Ozone = Protection = No sunburn!
Formation and Function of Ozone
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's talk about how ozone is formed. UV radiation can split molecular oxygen. Can anyone explain this process?

The UV rays break the O₂ molecules into individual oxygen atoms, right?

Exactly! Those free oxygen atoms can then combine with O₂ to form O₃. So, you can remember this process as 'UV + O₂ = O + O → O₃'. Anyone see why this is vital?

Because without it, we wouldn't have that shield against UV rays!

Spot on! The ozone layer acts like Earth's sunscreen. Remember: UV radiation = Ozone formation = Protection!
Depletion of the Ozone Layer
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Moving on, what has been causing depletion in the ozone layer?

Is it those chemicals like CFCs?

Yes! Chlorofluorocarbons, or CFCs, are the main culprits. They are found in various household products. Can anyone think of an example of a product that uses CFCs?

Aren't they in refrigerators?

That's right! Since the 1980s, CFC levels have decreased due to international agreements. Remember: CFCs = Bad for ozone = Solar protection.
International Agreements and Future of the Ozone Layer
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let’s look at what has been done to protect our ozone layer. Who can tell me what action was taken in 1987?

That was when the Montreal Protocol was signed, right?

Correct! This international agreement aimed to reduce CFC production. Why is this significant?

Because it shows how countries can work together to solve environmental problems!

Exactly! Together, we can protect our ozone layer. Remember: International action = Brighter future for Earth!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The ozone layer, formed from oxygen atoms, plays a key role in protecting life on Earth from damaging ultraviolet radiation. This section discusses how synthetic chemicals, particularly chlorofluorocarbons (CFCs), have led to significant depletion of the ozone layer, with historical actions such as the 1987 UNEP agreement aiming to mitigate this issue.
Detailed
Ozone Layer and How it is Getting Depleted
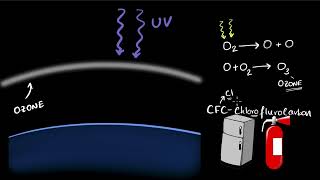
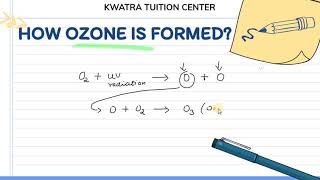
The ozone layer, composed of ozone (O₃) molecules formed from oxygen (O₂), is vital for life on Earth as it absorbs harmful ultraviolet (UV) radiation from the Sun. UV radiation can lead to serious health issues, including skin cancer. The formation of ozone occurs when UV radiation interacts with oxygen molecules:
- The process:
- UV radiation splits molecular oxygen (O₂) into free oxygen atoms (O).
- These atoms combine with O₂ to form ozone (O₃).
Since the 1980s, the amount of ozone in the atmosphere has sharply declined, mainly due to the use of chlorofluorocarbons (CFCs), which are used in refrigeration, aerosol sprays, and foam-blowing agents. The United Nations Environment Programme (UNEP) took action in 1987 to freeze CFC production, advocating for the adoption of CFC-free technologies worldwide. This effort aims to repair the ozone layer and protect ecosystems from the harmful effects of UV radiation.
Youtube Videos








Key Concepts
-
Ozone Layer: Essential for blocking UV radiation and protecting life on Earth.
-
CFCs: Human-made chemicals responsible for ozone layer depletion.
-
Montreal Protocol: An international agreement aimed at reducing ozone-depleting substances.
Examples & Applications
Example: CFCs are found in old refrigerators, leading to ozone depletion when released into the atmosphere.
Example: Increased UV radiation due to thinning ozone can lead to higher skin cancer rates.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Ozone high and CFCs low, keep the UV rays from making us glow!
Stories
Once there was a world where ozone danced above, shielding everything from harmful rays with love. But one day, CFCs came to play, threatening the ozone and turning night to day.
Memory Tools
Ozone means Only Zone for No Exposure (to UV).
Acronyms
O² = Oxygen, O³ = Ozone; Protect with O³!
Flash Cards
Glossary
- Ozone
A molecule made up of three oxygen atoms (O₃) that absorbs ultraviolet radiation.
- Chlorofluorocarbons (CFCs)
Synthetic substances used in refrigeration and aerosol sprays that deplete the ozone layer.
- Ultraviolet (UV) Radiation
Invisible rays from the sun that can cause skin cancer and other damage.
- Montreal Protocol
An international treaty aimed at reducing the use of substances that deplete the ozone layer.
Reference links
Supplementary resources to enhance your learning experience.
