Transport of Oxygen
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Oxygen Transport
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we'll explore how oxygen is transported in the body. Can anyone tell me how important oxygen is for our body?

Oxygen is essential for respiration! Our cells need it to produce energy.

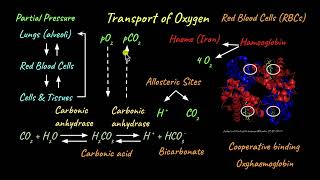
Exactly! Oxygen is necessary for cellular respiration, which generates energy. In humans, oxygen is primarily transported by red blood cells using a protein called hemoglobin.

What makes hemoglobin special for transporting oxygen?

Great question! Hemoglobin can bind to oxygen in a reversible manner. This means it can pick up oxygen in the lungs and release it in the tissues. Each hemoglobin molecule can carry up to four oxygen molecules.

So, how does it know when to release the oxygen?

It has to do with partial pressures. In areas of high pO2, like the lungs, hemoglobin binds to oxygen, while in areas with lower pO2, like tissues, it releases it.

Can we visualize this process?

Absolutely! We use an oxygen dissociation curve to visualize how hemoglobin's affinity for oxygen changes depending on pO2, pCO2, and other factors. Let's explore that next!
Factors Affecting Oxygen Transport
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s look at the factors affecting how hemoglobin binds and releases oxygen. Who can remind us of these factors?

I remember you mentioned pO2, pCO2, and temperature.

Exactly! In areas with high pO2, such as in the alveoli of the lungs, hemoglobin tends to bind oxygen more readily. However, in tissues where pO2 is lower, hemoglobin releases oxygen.

And what role does temperature play?

Good observation! Higher temperatures often cause hemoglobin to release oxygen more easily, which is crucial during exercise when tissues are warmer and have higher metabolic rates.

Does increasing pCO2 affect oxygen release too?

Yes! Increased pCO2 in tissues enhances the release of oxygen through a phenomenon known as the Bohr effect. This is beneficial because tissues producing more CO2 require more oxygen.

So, it's like a signal for hemoglobin to know when to release more oxygen?

Exactly! It’s all about the body’s needs. Now, let's summarize what we’ve learned about oxygen transport.
Oxygen Dissociation Curve
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

We’ve talked about various factors impacting hemoglobin's binding to oxygen. Now let’s look at the oxygen dissociation curve. Can anyone tell me what this is?

Isn't it a graph that shows how hemoglobin saturation changes with pO2?

Exactly! The curve is sigmoidal, meaning it has an S-shape. This reflects the cooperative binding aspect of hemoglobin; as one oxygen molecule binds, it makes it easier for others to bind.

And how does this curve help us understand the respiratory process?

Great point! During respiratory changes, the position of the curve shifts. If pCO2 levels rise or temperature increases, the curve shifts to the right—making it easier for hemoglobin to release oxygen in tissues.

So, this means our body adapts based on different conditions?

Precisely! The body is very adaptive. Let’s review everything shortly before moving on to related topics.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Oxygen transport in the body is mainly conducted by hemoglobin, which binds oxygen reversibly. This section highlights the factors influencing oxygen binding and the physiological significance of these processes in gas transport.
Detailed
The transport of oxygen is a vital physiological process wherein oxygen is provided to tissues for metabolic activities. Hemoglobin, an iron-containing protein in red blood cells, plays an essential role in this process by binding oxygen to form oxyhemoglobin. The binding affinity of hemoglobin for oxygen is largely influenced by the partial pressure of oxygen (pO2) in different body compartments, as well as additional factors such as pCO2 (carbon dioxide pressure), hydrogen ion concentration, and temperature. The oxygen dissociation curve illustrates how these factors affect the release and uptake of oxygen, demonstrating that oxygen is readily absorbed in the lungs and released in the tissues. This section mentions that under normal physiological conditions, 100 mL of oxygenated blood can deliver approximately 5 mL of oxygen to tissues.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Haemoglobin and Oxygen Binding
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Haemoglobin is a red coloured iron containing pigment present in the RBCs. O₂ can bind with haemoglobin in a reversible manner to form oxyhaemoglobin. Each haemoglobin molecule can carry a maximum of four molecules of O₂.
Detailed Explanation
Haemoglobin, found in red blood cells (RBCs), is crucial for oxygen transport. It can bind oxygen molecules to form oxyhaemoglobin. This binding is reversible, meaning that haemoglobin can release the oxygen when needed by the body's tissues. Each haemoglobin molecule has the capability to bind four oxygen molecules, enabling efficient oxygen transport.
Examples & Analogies
Think of haemoglobin as a delivery truck that carries oxygen to neighborhoods (tissues) in the city (the body). Just like a truck can load and unload packages, haemoglobin can pick up oxygen in the lungs and drop it off where it's needed, delivering four packages (oxygen molecules) at once.
Factors Influencing Oxygen Binding
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Binding of oxygen with haemoglobin is primarily related to partial pressure of O₂. Partial pressure of CO₂, hydrogen ion concentration and temperature are the other factors which can interfere with this binding.
Detailed Explanation
The amount of oxygen that binds to haemoglobin depends largely on the partial pressure of oxygen (pO₂). Higher pO₂ levels lead to increased oxygen binding. Other factors, such as the level of carbon dioxide (pCO₂), acidity (hydrogen ion concentration), and temperature, can also affect this binding. For instance, if CO₂ levels are high, it can lead to a release of oxygen from haemoglobin.
Examples & Analogies
Consider a sponge that absorbs water. The sponge absorbs more water when it’s dry (high pO₂) and releases it when it’s wet (high CO₂ levels). Similarly, haemoglobin picks up oxygen when conditions are right in the lungs and releases it in the tissues where CO₂ levels are high.
Oxygen Dissociation Curve
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A sigmoid curve is obtained when percentage saturation of haemoglobin with O₂ is plotted against the pO₂. This curve is called the Oxygen dissociation curve.
Detailed Explanation
The relationship between the percentage of hemoglobin saturated with oxygen and the partial pressure of oxygen is illustrated by a sigmoid (S-shaped) curve known as the oxygen dissociation curve. This curve is important for understanding how easily hemoglobin can bind or release oxygen under varying conditions.
Examples & Analogies
Imagine climbing a mountain. As you ascend, the air gets thinner (lower pO₂) and you find it harder to breathe (lower saturation of oxygen). The sigmoid curve reflects this experience – at certain heights, even with less oxygen, your body can still function well at lower saturation levels due to the curve's shape.
Conditions Favoring Oxygen Binding
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In the alveoli, where there is high pO₂, low pCO₂, lesser H+ concentration, and lower temperature, the factors are all favourable for the formation of oxyhaemoglobin, whereas in the tissues, where low pO₂, high pCO₂, high H+ concentration, and higher temperature exist, the conditions are favourable for dissociation of oxygen from the oxyhaemoglobin.
Detailed Explanation
In the lungs (alveoli), the conditions are optimal for hemoglobin to pick up oxygen (high pO₂ and low pCO₂). In contrast, in body tissues, where oxygen is utilized, conditions change—pO₂ drops while pCO₂ increases and acidity rises. These changes signal hemoglobin to release oxygen so it can be used in cellular respiration.
Examples & Analogies
Think of a restaurant where fresh food is delivered. In the 'kitchen' (lungs), there are plenty of ingredients (oxygen) to cook with, so the chefs (hemoglobin) are happy to take in as much as they can. Once the meal is prepared and served (in the body), the kitchen is busy cooking and uses up the ingredients, forcing the chefs to let go of the food they were holding onto.
Oxygen Delivery to Tissues
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
O₂ gets bound to haemoglobin in the lung surface and gets dissociated at the tissues. Every 100 ml of oxygenated blood can deliver around 5 ml of O₂ to the tissues under normal physiological conditions.
Detailed Explanation
Hemoglobin binds oxygen in the lungs (alveoli) where oxygen levels are high. As blood circulates and reaches tissues where oxygen is needed, the differing conditions encourage hemoglobin to release the oxygen. On average, 100 ml of oxygen-rich blood delivers about 5 ml of oxygen to active tissues, supporting their metabolic needs.
Examples & Analogies
Imagine a delivery service where each truck carries 5 packages of fresh produce (oxygen). As the truck reaches various neighborhoods (tissues), it drops off the packages (oxygen) that the residents need, ensuring that everyone gets the nourishment they require.
Key Concepts
-
Oxygen Transport: The process by which oxygen is carried through the bloodstream to tissues.
-
Hemoglobin: The protein responsible for oxygen transport.
-
Oxygen Dissociation Curve: Shows how hemoglobin saturation changes with varying pO2 levels.
Examples & Applications
When a person exercises, muscle cells produce more carbon dioxide and heat, leading to a rightward shift in the oxygen dissociation curve, facilitating oxygen release from hemoglobin.
In high altitudes, the lower pO2 levels can affect the efficiency of oxygen transport, prompting physiological adaptations.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Oxygen's flow through the blood, / Hemoglobin's ride in a red flood.
Stories
Imagine hemoglobin as a taxi driver that picks up passengers (oxygen) at the lung station and drops them off at tissue destinations, adjusting its route based on traffic (pCO2 and temperature levels).
Memory Tools
Think of 'HOT' for Factors: H for Hydrogen, O for Oxygen, T for Temperature impacting oxygen release.
Acronyms
Remember 'POET'
for pO2
for oxygen binding
for energy production
for tissue delivery.
Flash Cards
Glossary
- Hemoglobin
An iron-containing protein in red blood cells that binds oxygen for transport.
- Oxyhemoglobin
The complex formed when oxygen binds to hemoglobin.
- pO2
Partial pressure of oxygen, a measure of oxygen concentration in a given environment.
- pCO2
Partial pressure of carbon dioxide, a measure of carbon dioxide concentration in a given environment.
- Oxygen Dissociation Curve
A graphical representation showing the relationship between pO2 and hemoglobin saturation with oxygen.
Reference links
Supplementary resources to enhance your learning experience.
