Differential Extraction
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Differential Extraction
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Good morning everyone! Today, we are going to discuss differential extraction, a fundamental technique in organic chemistry. Can anyone tell me what they think 'differential extraction' means?

Is it something to do with separating compounds from mixtures?

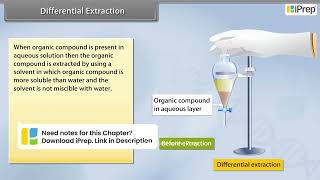
Exactly, Student_1! Differential extraction is a method used to separate an organic compound from an aqueous solution by using a suitable organic solvent. Why do you think we need to use a solvent for the separation?

So the compound can dissolve better in the solvent compared to water?

Right! The key here is that the compound must have a higher solubility in the organic solvent than in the water. This is crucial for the extraction process.

But, how do we actually separate the layers once we've mixed them?

Great question! After shaking the mixture, we'll let it sit so that two distinct layers form. The organic layer usually sits on top or below the aqueous layer, depending on the densities. Using a separatory funnel, we can drain one layer from the other.

What if the compound dissolves equally in both solvents?

If the compound has similar solubility in both, we might need to use more advanced techniques, like continuous extraction, to improve the yield. Let's summarize: differential extraction allows us to separate a compound from water using a solvent where it has higher solubility. Remember, the solvent selection is vital!
Practical Applications of Differential Extraction
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand the basics of differential extraction, let’s talk about where this technique is commonly applied. Can anyone think of scenarios in a laboratory where differential extraction might be useful?

Extracting essential oils from plants could be one!

Absolutely! Extracting essential oils is a great example. It's also used in purifying organic compounds synthesized in reactions. These compounds typically need to be purified before further experimental use.

Are there any limitations to this method?

Yes, limitations do exist. For instance, if the organic compound is only slightly soluble in the solvent, a larger volume will be needed for extraction, which can be inefficient.

So, continuous extraction would be a solution here?

Exactly, Student_3! Continuous extraction allows repeated extractions using the same solvent, which can increase the yield of the compound. Let’s recap: differential extraction is important for purifying substances in chemistry, but choosing the right solvent is key!
Steps in Differential Extraction
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's dive into the steps for performing differential extraction. First, can anyone tell me the initial action we need to take?

We need to mix the organic solution with the solvent, right?

Correct! Once you mix the compound in the solvent, what comes next?

Shake it vigorously to mix them well?

Good job! After shaking, it's essential to let the mixture settle. Why is that?

So we can see the different layers more clearly?

Exactly! After settling, we’ll drain the bottom layer using a separatory funnel. Finally, what must we do with the organic solvent?

We need to evaporate it to retrieve our compound.

Right! This method allows us to isolate our desired compound effectively. Through differential extraction, we ensure that the compound of interest is removed from water, enabling further study or application. Remember, proper technique and solvent selection are crucial!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section discusses differential extraction, emphasizing its importance in separating organic compounds from water. It outlines the process of extracting compounds into a separatory funnel and the conditions required for effective extraction.
Detailed
Differential Extraction
Differential extraction is a versatile technique employed in organic chemistry for the separation and purification of compounds from aqueous solutions. This method exploits the varying solubility of compounds in different solvents, allowing for efficient isolation of organic substances.
Key Concepts of Differential Extraction
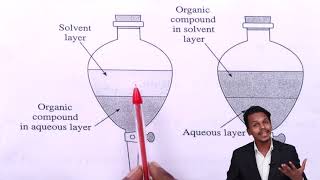
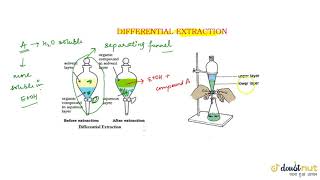
- Basic Principle: The process involves shaking an organic compound present in an aqueous medium with an immiscible organic solvent in which it is more soluble. This results in the formation of two distinct layers that can be easily separated.
- Solvent Selection: Choosing an appropriate organic solvent is critical. The organic solvent must have a higher affinity for the compound of interest compared to water to ensure effective extraction.
- Separation Process: Once the layers form, the organic solvent can be removed through a separatory funnel. If the compound is less soluble in the solvent, it may require continuous extraction where the same solvent is used multiple times to achieve a higher yield of the desired compound.
Significance in Organic Chemistry
Differential extraction is crucial for purifying organic compounds synthesized in laboratory settings or isolated from natural sources. It facilitates the removal of impurities, thereby enhancing the quality and yield of the desired compounds for further analysis or application.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Overview of Differential Extraction
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When an organic compound is present in an aqueous medium, it is separated by shaking it with an organic solvent in which it is more soluble than in water.
Detailed Explanation
Differential extraction is a technique used in organic chemistry to separate compounds based on their solubility differences in two immiscible solvents, commonly water and an organic solvent. The process involves shaking an aqueous solution containing the organic compound with an organic solvent where the compound has a higher solubility. This causes the organic compound to move from the aqueous phase into the organic phase, thus effectively separating it from water.
Examples & Analogies
Think of a fruit salad where you have peaches in water. If you want to separate the peaches, you can use a thicker syrup where the peaches dissolve better than in water. Just as you would pour the syrup to get the peaches out, in chemistry, we use this concept to extract compounds into different layers using organic solvents.
Conditions for Successful Extraction
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The organic solvent and the aqueous solution should be immiscible with each other so that they form two distinct layers which can be separated by a separatory funnel.
Detailed Explanation
For differential extraction to be effective, the organic solvent must not mix with the water-based solution. This immiscibility allows the two solvents to form separate layers. When the mixture is shaken, the organic solvent will selectively dissolve the organic compound, and after settling, the layers can be separated. A common example of immiscible solvents is water and oil, which can easily be poured into separate containers.
Examples & Analogies
Imagine making a salad dressing with oil and vinegar. When you shake them together, they mix temporarily, but as soon as you stop shaking, the oil and vinegar separate into two distinct layers because they do not mix. This is similar to how organic solvents need to behave in chemical extractions.
Separation Process
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The organic solvent is later removed by distillation or by evaporation to get back the compound.
Detailed Explanation
Once the organic compound has been transferred to the organic layer, the solvent needs to be removed to isolate the compound. This can be achieved through distillation, where the solvent is heated until it vaporizes and is then condensed back into liquid form, leaving the organic compound behind. Alternatively, evaporation can be used where the solvent is allowed to evaporate naturally, leaving the compound to crystallize or solidify.
Examples & Analogies
If you’ve ever made sugar syrup, you know that after boiling the water with sugar, you can let the mixture cool and evaporate, which leaves behind the sugar crystals. Similarly, when we evaporate the solvent after extraction, the desired compound is left behind, purified and ready for use.
Continuous Extraction Technique
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If the organic compound is less soluble in the organic solvent, a very large quantity of solvent would be required to extract even a very small quantity of the compound. The technique of continuous extraction is employed in such cases.
Detailed Explanation
In cases where an organic compound is not easily soluble in the chosen organic solvent, more solvent is required to achieve effective extraction. Continuous extraction addresses this challenge by repeatedly using the same solvent to extract the compound. This method allows for efficient extraction over time without the need for a large volume of solvent at once, thus enhancing yield.
Examples & Analogies
Think about trying to squeeze lemon juice from a lemon. If you just try to squeeze it once, you might only get a few drops. But if you keep squeezing it repeatedly, you get a lot more juice. Continuous extraction works in a similar way, systematically pulling out the organic compound until there’s little left.
Key Concepts
-
Basic Principle: The process involves shaking an organic compound present in an aqueous medium with an immiscible organic solvent in which it is more soluble. This results in the formation of two distinct layers that can be easily separated.
-
Solvent Selection: Choosing an appropriate organic solvent is critical. The organic solvent must have a higher affinity for the compound of interest compared to water to ensure effective extraction.
-
Separation Process: Once the layers form, the organic solvent can be removed through a separatory funnel. If the compound is less soluble in the solvent, it may require continuous extraction where the same solvent is used multiple times to achieve a higher yield of the desired compound.
-
Significance in Organic Chemistry
-
Differential extraction is crucial for purifying organic compounds synthesized in laboratory settings or isolated from natural sources. It facilitates the removal of impurities, thereby enhancing the quality and yield of the desired compounds for further analysis or application.
Examples & Applications
Example of extracting caffeine from coffee using differential extraction.
Isolating essential oils from plant material using organic solvents.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Extract the best, let it rest, find the layers to be blessed!
Stories
Imagine a chemist who wanted to get the essence of flowers. They take the petals and put them into a jar with water and oil, knowing the oil will pull out the flower's perfume. With patience, they wait and watch the liquid separate — the beauty of extraction!
Memory Tools
SPE - Shake, Separate, Evaporate - remember the steps of differential extraction.
Acronyms
D.E.S.I.G.N - Differential Extraction Simplifies In General Notation (for remembering the process).
Flash Cards
Glossary
- Differential Extraction
A separation technique used to isolate a compound from an aqueous solution by shaking it with an organic solvent in which it has higher solubility.
- Aqueous Solution
A solution in which water is the solvent.
- Separatory Funnel
A laboratory glassware tool used to separate mixtures of liquids based on differences in density.
- Immiscible
Incapable of mixing or being mixed; refers to liquids that do not mix to form a homogeneous solution.
- Continuous Extraction
A method used to extract a compound where the same solvent is used repeatedly to increase the yield.
Reference links
Supplementary resources to enhance your learning experience.
