THE INTERNATIONAL SYSTEM OF UNITS
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to SI Units
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're delving into the International System of Units, or SI. Who can tell me why having a standardized system for units is important?

It's important for scientists across the world so they can communicate findings without confusion.

Exactly! Different systems like CGS and MKS created challenges. SI helps unify measurements. Can anyone share what some of these base units are?

Length, mass, time, and electric current are some of them!

Right! Let’s remember those with the acronym **LMTEC**—Length, Mass, Time, Electric Current. Any questions about the significance of these base units?

Why do we need a base unit for angles?

Great question! Angles are vital in physics and engineering, hence we measure them in radians and steradians. Let's summarize: SI unifies measurement, making it easy for global communication!
Derived Units in SI
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

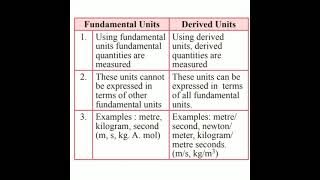
Now let's look at derived units. Can someone explain what derived units are?

They are units formed by combining base units.

Exactly! For instance, the unit of velocity is derived from length and time. Remember the formula **v = d/t**, where velocity (v) equals distance (d) over time (t). What are the SI units for velocity?

Metres per second (m/s)!

Correct! Remember also that derived units can have specific names, like Newton for force. Now let’s summarize derived units: they emerge from base units and help express more complex physical quantities.
Conversion within SI
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Conversions make our work simpler. Why might SI's decimal system be preferable for conversions?

Because it allows for straightforward decimal shifts, making calculations easier!

Exactly right! This is why SI is so convenient in scientific calculations. Can anyone think of a scenario in which SI units help avoid errors?

When comparing scientific data from different countries—it prevents miscommunication due to unit discrepancies!

Well said! Each SI unit has defined standard symbols, too, like 'm' for metre. To summarize: SI facilitates easy conversions and minimizes errors in international science.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The SI system provides a standardized method for expressing physical quantities through seven base units—length, mass, time, electric current, thermodynamic temperature, amount of substance, and luminous intensity. Derived units are formed from these base units, ensuring consistency and ease of use in various scientific fields.
Detailed
Detailed Summary of the International System of Units
The International System of Units (SI), known as Système Internationale d'Unités, is the modern form of the metric system and is widely used in scientific and technical disciplines. Initially, different countries used various systems like CGS (centimetre-gram-second), FPS (foot-pound-second), and MKS (metre-kilogram-second) for measurements. This diversity was problematic for international communication and standardization.
To streamline measurements, the SI system was developed, which adopts a standard set of units universally accepted. The SI consists of seven base units:
- Length - Metre (m): Defined using the speed of light.
- Mass - Kilogram (kg): Defined using the Planck constant.
- Time - Second (s): Based on the hyperfine transition frequency of caesium-133.
- Electric Current - Ampere (A): Defined by the elementary charge.
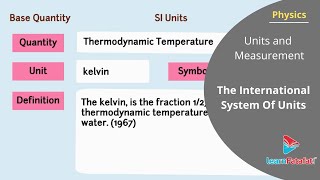
- Thermodynamic Temperature - Kelvin (K): Defined using the Boltzmann constant.
- Amount of Substance - Mole (mol): Contains exactly the Avogadro number of entities.
- Luminous Intensity - Candela (cd): Based on specific radiation.
Beyond these, SI also includes plane and solid angles, measured in radians (rad) and steradians (sr), respectively. The simplicity of the SI system stems from its base-10 structure, facilitating conversions between units. As technological advancement continues, the definitions of these units are refined to maintain accuracy and consistency in measurements.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Historic Measurement Systems
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In earlier time scientists of different countries were using different systems of units for measurement. Three such systems, the CGS, the FPS (or British) system and the MKS system were in use extensively till recently.
Detailed Explanation
Before the adoption of a standard system, scientists used various measurement systems. The CGS (centimetre-gram-second) system was based on centimetres for length, grams for mass, and seconds for time. The FPS (foot-pound-second) system used feet, pounds, and seconds, while the MKS (metre-kilogram-second) system employed metres, kilograms, and seconds. These varying systems often complicated communication and calculations in scientific work.
Examples & Analogies
Imagine trying to buy fabric: if one store measures in yards and another in meters, it could lead to confusion and mistakes. This is similar to how scientists faced challenges when using different systems of measurement.
International System of Units (SI)
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The system of units which is at present internationally accepted for measurement is the Système Internationale d’ Unites (French for International System of Units), abbreviated as SI. The SI, with a standard scheme of symbols, units and abbreviations, developed by the Bureau International des Poids et measures (The International Bureau of Weights and Measures, BIPM) in 1971 were recently revised by the General Conference on Weights and Measures in November 2018.
Detailed Explanation
The SI is the globally accepted system for measurement that provides consistency and clarity. It simplifies scientific communication by standardizing units of measurement, which were previously inconsistent. Established in 1971, it was revised to ensure that definitions reflect the latest scientific advancements, making it relevant and accurate.
Examples & Analogies
Using SI units is like having a universal language; just as language facilitates easier communication between people, SI units enable scientists worldwide to share and understand results without confusion.
Base Units of the SI System
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In SI, there are seven base units as given in Table 1.1. Besides the seven base units, there are two more units that are defined for (a) plane angle d θ as the ratio of length of arc d s to the radius r and (b) solid angle dΩ as the ratio of the intercepted area d A of the spherical surface, described about the apex O as the centre, to the square of its radius r. The unit for plane angle is radian with the symbol rad and the unit for the solid angle is steradian with the symbol sr. Both these are dimensionless quantities.
Detailed Explanation
The SI system comprises seven fundamental units: metre (length), kilogram (mass), second (time), ampere (electric current), kelvin (thermodynamic temperature), mole (amount of substance), and candela (luminous intensity). Additionally, radians and steradians measure angles, although they do not have dimensions. The definitions of these units are precise, which allows for standard measurements across various fields of science and technology.
Examples & Analogies
Think of the SI base units as the building blocks of measurement. Just as you need bricks, cement, and wood to build a house, these base units serve as the foundation for all scientific measurements, creating a strong and shared framework for discovery.
Base Quantities and Their Definitions
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Base quantity Name Symbol Definition Length metre m The metre, symbol m, is the SI unit of length. It is defined by taking the fixed numerical value of the speed of light in vacuum c to be 299792458 when expressed in the unit m s–1, where the second is defined in terms of the caesium frequency ∆νcs. Mass kilogram kg The kilogram, symbol kg, is the SI unit of mass. It is defined by taking the fixed numerical value of the Planck constant h to be 6.62607015×10–34 when expressed in the unit J s, which is equal to kg m2 s–1, where the metre and the second are defined in terms of c and ∆νcs. Time second s The second, symbol s, is the SI unit of time. It is defined by taking the fixed numerical value of the caesium frequency ∆νcs, the unperturbed ground-state hyperfine transition frequency of the caesium-133 atom, to be 9192631770 when expressed in the unit Hz, which is equal to s–1.
Detailed Explanation
The exact definitions of the base quantities provide a scientific standard. For instance, the metre is defined by the speed of light, which is a universal constant, assuring that the unit remains consistent. Similarly, the kilogram is tied to the Planck constant, and the second is based on a specific property of the caesium atom. This reliance on fundamental constants ensures that the measures are stable over time.
Examples & Analogies
Using these definitions is like using a specific recipe to make a dish. Just as each ingredient must be measured precisely to achieve the desired flavor, using defined constants guarantees that measurements are accurate and universally understood across the scientific community.
Key Concepts
-
International System of Units (SI): A globally accepted system for measurement based on seven base units.
-
Base Units: The fundamental measurements of length, mass, time, electric current, thermodynamic temperature, amount of substance, and luminous intensity.
-
Derived Units: Units created by combining base units to express more complex physical quantities.
-
Importance of Standardization: The necessity for a universal measurement system to facilitate scientific communication.
Examples & Applications
An example of a derived unit is velocity, which is measured in metres per second (m/s).
Another derived measurement is energy, often represented in joules (J), which can be expressed as kg·m²/s².
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
SI units are oh so neat, measuring lengths, mass, and heat!
Stories
Imagine a scientist who travels the world, and everywhere they go, they measure things in m, kg, and s. This scientist feels great knowing everyone understands them because of the SI system.
Memory Tools
Remember LMTCE for the seven base units: Length, Mass, Time, Electric current, Thermodynamic temperature, Amount of substance, and Luminous intensity.
Acronyms
Use the acronym BUD (Base Units Derived) to remember that derived units come from base units.
Flash Cards
Glossary
- Système Internationale d'Unités (SI)
The modern form of the metric system, adopted for scientific measurements.
- Base Units
Fundamental quantities that represent the dimensions of physical concepts.
- Derived Units
Units formed by combinations of base units.
- Radian
Unit of measure for angles, defined as the ratio of the length of an arc to its radius.
- Steradian
Unit of measure for solid angles, defined as the ratio of the intercepted area to the square of the radius.
Reference links
Supplementary resources to enhance your learning experience.
