Cement
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Cement
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will explore cement, the binding material essential for concrete, which is crucial in construction. Can anyone tell me why cement is considered important?

I think it holds all the ingredients together.

Exactly! Cement binds aggregates and water. Now, what is the main type of cement we use?

Ordinary Portland Cement, right?

Correct! We often refer to it as OPC. It's critical to understand the chemical composition, which we'll discuss next.
Chemical Composition of Cement
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Cement consists of several chemical compounds. Can anyone name one?

Tricalcium silicate, C₃S?

Great! C₃S provides early strength. Let's remember it with the mnemonic **‘C3 Strong’**. How about another compound?

Dicalcium silicate, C₂S?

Exactly! C₂S contributes to long-term strength. Remember, high percentages of C₃S help during the initial setting.

What role does C₃A play?

C₃A influences setting time and generates heat during hydration. Let's move on to hydration.
Heat of Hydration
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

When cement interacts with water, it generates heat. Can anyone describe what happens during this process?

It’s called heat of hydration!

Right! This reaction is exothermic and can lead to issues like cracking if not controlled. High heat in massive structures needs techniques to mitigate this risk.

What can we do to control that?

Good question! Cooling techniques or using slow-setting cements can help. Remember, managing heat is crucial for durability.
Quality Tests for Cement
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To ensure cement's quality, various tests are conducted. Can anyone name one?

The fineness test?

Yes! Fineness affects the surface area, and we'd use methods like Blaine’s air permeability. What’s another test?

The initial and final setting time tests?

Absolutely! These tests are crucial for understanding workability and setting behavior. Let’s summarize the importance of quality tests.

In summary, understanding cement's chemical composition, heat of hydration, and rigorous testing is essential for producing quality concrete.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Cement acts as the binding agent in concrete, primarily composed of Ordinary Portland Cement (OPC). The chemical composition influences its physical properties, setting time, and hydration process, which are vital for civil engineering applications.
Detailed
Cement in Concrete
Cement is a pivotal ingredient in concrete, serving as the binding material that holds all other components together—specifically aggregates and water. The most commonly utilized type of cement is Ordinary Portland Cement (OPC). This section delves into the chemical composition of cement, its hydration process, its structure after setting, and the various quality control tests that ensure its effectiveness in concrete production. The heat of hydration is a key aspect, as it can affect the strength and durability of concrete under different environmental conditions. Understanding these fundamental characteristics of cement is essential for engineers to optimize concrete formulations for specific construction needs.
Youtube Videos




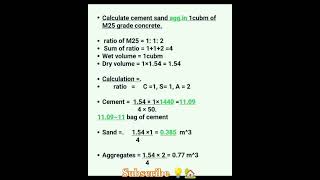





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Cement
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Cement is the binding material that binds the other ingredients together upon hydration. The most commonly used type of cement in concrete is Ordinary Portland Cement (OPC).
Detailed Explanation
Cement is a crucial component in making concrete. It acts as a glue that holds together other materials, such as aggregates (sand and gravel) and water. When cement is mixed with water, it undergoes a chemical reaction called hydration, which allows it to harden and bind the other ingredients together. Ordinary Portland Cement (OPC) is the most commonly used type of cement in construction due to its strength and durability.
Examples & Analogies
Think of cement like glue when building with LEGO blocks. Just as glue helps to hold the blocks together, cement holds the concrete components in place, providing strength and integrity to the structures we build.
Chemical Composition of Cement
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The primary chemical compounds found in Portland cement are:
- Tricalcium Silicate (C₃S) - 40–60%: Provides early strength
- Dicalcium Silicate (C₂S) - 15–30%: Provides long-term strength
- Tricalcium Aluminate (C₃A) - 5–12%: Influences setting time, heat of hydration
- Tetracalcium Aluminoferrite (C₄AF) - 6–10%: Provides color and reduces cost
Other minor compounds include alkalis, magnesium oxide, and gypsum (added to control the setting time).
Detailed Explanation
Portland cement contains several key chemical compounds that contribute to its strength and setting properties. Tricalcium silicate (C₃S) is important for gaining strength quickly, while dicalcium silicate (C₂S) contributes to strength over a longer period. Tricalcium aluminate (C₃A) affects the rate at which the cement sets and the heat produced during hydration, whereas tetracalcium aluminoferrite (C₄AF) influences the color of cement and helps reduce production costs. Gypsum is added to manage the setting time of the cement.
Examples & Analogies
Imagine baking a cake where each ingredient contributes differently. The flour is like C₂S, which helps the cake rise over time, while baking powder (similar to C₃A) makes it rise quickly. Each ingredient is essential for the final product, just as the chemical compounds in cement are necessary for optimal performance.
Heat of Hydration
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When cement reacts with water, an exothermic chemical reaction known as hydration occurs, releasing heat. This is called the heat of hydration.
- C₃A and C₃S contribute most to the early heat generation.
- High heat of hydration can lead to cracking in massive concrete structures unless controlled by cooling techniques or slow-setting cements.
Detailed Explanation
Hydration is the chemical process where cement reacts with water and releases heat. This heat generation, called the heat of hydration, is crucial for the hardening of concrete. However, excessive heat can be problematic, especially in large structures, as it may cause the concrete to crack. Engineers may use cooling methods or select slow-setting cements to manage this heat effectively and prevent damage.
Examples & Analogies
Consider how a large pile of compost generates heat as it decomposes. Just like that heat can be beneficial for decomposition, the heat from cement hydration is necessary for concrete hardening. But if it gets too hot, it can lead to problems, much like how too much heat in composting can cause odors or burning out materials.
Structure of Hydrated Cement
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The hydration products include:
- Calcium Silicate Hydrate (C-S-H): Responsible for strength.
- Calcium Hydroxide (CH): Byproduct, contributes little to strength.
- Ettringite: Forms from C₃A and gypsum; affects setting and early strength.
- Monosulfate: Forms after ettringite transformation in later stages.
Detailed Explanation
During the hydration process, cement produces several compounds that affect how strong the concrete becomes. Calcium Silicate Hydrate (C-S-H) is the main product responsible for the concrete's strength. Calcium Hydroxide (CH) is produced as a byproduct during hydration but does not contribute much to strength. Ettringite affects how well the cement sets early on, while monosulfate is an advanced product that forms as the concrete continues to cure.
Examples & Analogies
Imagine a garden growing from seeds. The healthy plants that grow are like C-S-H, providing strength and beauty to the garden. The leftover soil, which doesn’t contribute to growth, can be likened to Calcium Hydroxide. Both are present, but only one truly supports the growth of your beautiful plants.
Physical Tests on Cement
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
To ensure quality and suitability for construction, the following tests are performed on cement:
- Fineness test (e.g., Blaine’s air permeability method)
- Standard consistency test
- Initial and final setting time
- Compressive strength of cement mortar cubes
- Soundness test (e.g., Le-Chatelier method)
- Specific gravity test.
Detailed Explanation
Quality assurance for cement includes various physical tests. The fineness test measures how fine the cement particles are, which affects hydration speed. The standard consistency test determines the amount of water needed for a consistent mix. Setting time tests check how long it takes for the cement to begin hardening and fully harden, which is crucial for construction schedules. Compressive strength tests ensure the cement can withstand forces, while soundness tests check for expansion that could cause cracking. Finally, specific gravity tests help assess the weight and density of the cement.
Examples & Analogies
Think of baking cookies. You wouldn’t just throw everything together without checking things like the flour's texture (fineness), how well it sticks when mixed (consistency), or how long it takes your cookies to bake (setting times). Similarly, proper testing ensures that cement will perform safely and effectively in construction.
Key Concepts
-
Chemical Composition: Cement's properties depend on its chemical compounds, primarily C₃S, C₂S, C₃A, and C₄AF.
-
Hydration: The reaction of cement with water releases heat, influencing setting and strength.
-
C-S-H: Calcium Silicate Hydrate is a product of hydration that greatly affects the concrete's strength.
Examples & Applications
Ordinary Portland Cement is commonly used for constructing buildings and pavements.
High-performance concrete often incorporates specific types of cement to enhance workability and durability.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Cement holds things tight, aggregates unite, in concrete's might!
Stories
Imagine a baker mixing flour (aggregates), water, and a special ingredient (cement) to create a dough that hardens into bread (concrete). Each ingredient must be just right for the bread to rise perfectly!
Memory Tools
For hydration, think H.E.A.T.: Heat, Exothermic, Actions with water, Time to set.
Acronyms
Use **C.A.S.H.** to remember
Cement - Aggregates - Strength - Hydration.
Flash Cards
Glossary
- Cement
The binding material in concrete that hardens when mixed with water and aggregates.
- Ordinary Portland Cement (OPC)
The most commonly used type of cement in concrete.
- Heat of Hydration
The heat generated from the exothermic chemical reaction when cement contacts water.
- C₃S (Tricalcium Silicate)
A compound in cement that provides early strength.
- C₂S (Dicalcium Silicate)
A compound in cement that contributes to long-term strength.
- C₃A (Tricalcium Aluminate)
A compound in cement that influences setting time and heat generation during hydration.
- CSH (Calcium Silicate Hydrate)
The main hydration product responsible for the strength of concrete.
Reference links
Supplementary resources to enhance your learning experience.
