Impact of Combustion on Air Quality
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Combustion Pollutants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're discussing combustion and its impact on air quality. Can anyone tell me what kind of pollutants might come from combustion processes?

I think gases like carbon dioxide and smoke come from burning fuel.

Exactly! Those are major products of combustion. In addition, we often see nitrogen oxides or NOx, sulfur dioxide, and various particulate matters generated. Can someone tell me why these pollutants are concerning?

They can make the air unhealthy to breathe, right?

Correct! They can lead to respiratory issues and other health problems. Remember, the acronym NOx stands for nitrogen oxides, a key player in air pollution. What about particulate matter? Can anyone elaborate on that?

Particulate matter is basically tiny solid particles that can be inhaled?

Well put! They can also include liquid droplets. Let's summarize: combustion not only emits CO2 but also NOx, SO2, and particulate matter—each significant for air quality.
Criteria Pollutants and Monitoring
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's delve into criteria pollutants. Why do you think they are referred to as criteria pollutants?

Maybe because they set criteria for air quality monitoring?

Exactly right! These pollutants are monitored for regulations to ensure safety. Can anyone mention some examples of criteria pollutants?

I remember NOx, SO2, and particulate matter. Are there others?

Yes! Unburnt hydrocarbons are also significant. Including these in monitoring helps us understand pollution sources better. Who can tell me about the importance of monitoring?

It helps keep track of what’s in the air, so we can take action if it’s harmful.

Great insight! Monitoring is crucial to protect our health and environment. So, remember to think about the relationship between pollutant levels and our quality of life.
Regulatory Bodies and Societal Responsibility
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's shift gears and consider how society responds to combustion emissions. Who is responsible for regulating combustion emissions?

Is it the government or specific agencies?

Spot on! Agencies like the Central Pollution Control Board enforce regulations to help ensure that we maintain our air quality standards. But whose responsibility is it really?

It’s everyone's, right? We all contribute to pollution!

Absolutely! Each individual and sector has a role to play in reducing our carbon footprint and pollution. What actions can we take?

Driving less and using public transport can help!

Excellent point! Fostering awareness and taking collective action is essential, and regulatory bodies require your feedback to improve policies.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section explores how combustion processes release numerous pollutants such as NOx, SO2, and particulate matter into the atmosphere, negatively affecting air quality and public health. It discusses the importance of monitoring these pollutants for regulatory purposes.
Detailed
Impact of Combustion on Air Quality
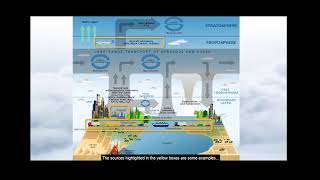
This section discusses the impact of combustion as a major source of air pollution. Combustion processes release several pollutants, including nitrogen oxides (NOx), sulfur dioxide (SO2), ozone, carbon monoxide, and particulate matter (PM). The paragraph begins by defining aerosols as a mixture of solid and liquid particles dispersed in gas, which includes the common ambient air. It emphasizes the fact that air pollution cannot be understood without assessing these aerosol components.
Key Points:
- Aerosols: Defined as mixtures of solid or liquid particles in air, primarily impacting health when inhaled.
- Combustion as a Source: The main contribution to air pollution arises from combustion, especially from transportation and industrial activities.
- Criteria Pollutants: Focus on major pollutants such as NOx, SO2, particulate matter, and unburnt hydrocarbons, which have significant regulatory importance.
-
Monitoring and Regulation: The importance of regulatory standards for ambient air quality and the impact of pollutants on human health is discussed, accentuating the role of regulatory bodies in mitigating these issues.

Significance:
Understanding the complex interplay of combustion processes and air quality is paramount for informing public policy and regulating emissions to protect environmental and human health.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
The Contribution of Combustion to Air Pollution
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So when we say the air is polluted, we need some handle on that. We cannot just say air is polluted. The biggest contributor to air pollution is 'combustion' that’s the biggest activity where something is converted and an exhaust is released, okay.
Detailed Explanation
This chunk discusses the primary sources of air pollution, which is combustion. Combustion refers to the process where fuels are burned to produce energy, such as in vehicles, factories, and power plants. This process releases pollutants into the air, indicating that monitoring air quality must prioritize understanding combustion impacts.
Examples & Analogies
Think of a campfire. When you burn wood, it produces smoke that can irritate your eyes and lungs. In the larger context, cities with heavy traffic have a 'campfire effect' where countless vehicles are 'burning fuel,' releasing smoke and other harmful pollutants into the air.
Major Pollutants from Combustion
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The pollutants that are coming out from combustion include NOx, SO2, ozone, particulate matter, and carbon monoxide.
Detailed Explanation
This part details specific pollutants emitted during combustion. NOx (nitrogen oxides) and SO2 (sulfur dioxide) are gases that can cause respiratory problems and contribute to acid rain. Ozone at ground level is harmful and can exacerbate asthma. Particulate matter consists of tiny particles that can penetrate the lungs and enter the bloodstream, while carbon monoxide is a toxic gas that can be fatal in high concentrations.
Examples & Analogies
Imagine a busy highway during rush hour. The exhaust from all the cars contains NOx and particulate matter. If you were to inhale deeply, you might feel short of breath—this is similar to how these pollutants can affect people's health.
The Phase-Out of Leaded Fuel
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Long back when this criteria pollutants started, there used to be another compound called lead, which is not there in most of the list now. Lead used to be a significant part of petrol.
Detailed Explanation
The environmental regulations have evolved, leading to the phase-out of leaded petrol, which was once a major pollutant. Lead was used as an anti-knocking agent in gasoline. Its removal helps reduce health risks associated with lead exposure, particularly for children who are more susceptible to its harmful effects.
Examples & Analogies
Consider a toy that was once painted with lead-based paint. Now, many parents check to ensure their children's toys are lead-free to avoid health risks. Similarly, removing lead from fuel has been a necessary upgrade to protect public health.
Unburnt Hydrocarbons
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Sometimes fuel does not combust properly, leading to unburnt hydrocarbons being released, which can condense into harmful compounds.
Detailed Explanation
This segment highlights unburnt hydrocarbons that result from incomplete combustion. When fuel doesn't burn completely, it can evaporate and create harmful vapors. These unburnt hydrocarbons can contribute to smog and respiratory issues, and are often present in emissions from older or poorly maintained vehicles.
Examples & Analogies
Think of when you cook food on a gas stove. If the flame is weak, your food might burn on the outside while remaining uncooked on the inside. This imperfect cooking is akin to how fuel burns—if it's not done completely, it produces waste (in this case, harmful hydrocarbons) instead of energy.
The Importance of Regulatory Monitoring
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If you find a lot of pollutants during air sampling, it's important to monitor these from time to time, leading them to become criteria pollutants.
Detailed Explanation
This chunk emphasizes the need for ongoing monitoring of air pollutants. Regulatory bodies conduct air sampling to assess pollution levels. If certain pollutants are found frequently and in high concentrations, they are declared as criteria pollutants that need to be regulated to protect public health.
Examples & Analogies
It's like going to a doctor for a check-up. If tests show high cholesterol, the doctor advises on dietary changes. Similarly, regular monitoring reveals pollution levels, allowing for necessary actions/recommendations to keep air quality safe.
Key Concepts
-
Aerosols are mixtures of solid and liquid particles in air that can affect health.
-
Combustion is the major source of pollutants like NOx, SO2, and particulate matter.
-
Criteria pollutants are monitored for regulatory compliance to safeguard air quality.
Examples & Applications
Vehicles emitting exhaust gases including NOx and particulate matter into urban air.
Industrial processes burning fossil fuels that release SO2 and unburnt hydrocarbons.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In the air, pollutants mix, from fuel that's burned, we get the kicks.
Stories
Once, a city full of cars believed they had a clean fare. But combustion let out NOx and soot, polluting their precious air.
Memory Tools
Remember 'Noble Souls Don't Always Unite', which stands for NOx, SO2, PM, Unburnt hydrocarbons.
Acronyms
Acronym 'CUP' for remembering Combustion, Unburnt hydrocarbons, and Pollutants.
Flash Cards
Glossary
- Aerosol
A mixture of fine solid particles or liquid droplets suspended in a gas.
- Combustion
A chemical reaction between a fuel and an oxidant that produces heat and light, leading to the release of various pollutants.
- Criteria pollutants
Pollutants that are regulated by air quality standards owing to their potential adverse effects on health and the environment.
- Particulate Matter (PM)
A complex mixture of tiny solid particles and liquid droplets found in the air.
- Nitrogen oxides (NOx)
Gases produced from combustion processes that contribute to air pollution and smog.
- Sulfur dioxide (SO2)
A gas produced from burning fossil fuels, which can cause health issues and contribute to acid rain.
- Unburnt hydrocarbons
Fuel components that were not completely burned during combustion, which can contribute to air pollution.
Reference links
Supplementary resources to enhance your learning experience.
