Concept of KOC and Partitioning
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to KOC
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's start by understanding KOC, which is the partition coefficient between organic carbon and water. This concept is essential for how we assess the behavior of chemicals in the environment, particularly their adsorption to organic materials. What do you think is a potential significance of KOC in environmental science?

Maybe it helps us understand how pollutants move in soil and water?

Exactly! KOC helps us predict how chemicals will behave in different environments and how quickly they might degrade. Remember, KOC helps classify chemicals based on their affinity for organic carbon—a high KOC means strong binding.

What if different types of organic carbon might change the KOC value?

Great question! Variability in organic carbon can indeed lead to different KOC values, which is why understanding the source and composition is crucial.
Deriving KOC
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's delve deeper into how we derive KOC. Can someone explain what goes into calculating it?

Is it just the concentration of the chemical in organic carbon versus water?

Yes, but normalized to account for the mass of organic carbon! Typically, KOC = mass of chemical A per mass of OC divided by mass of chemical A in water. How do we plan to measure these values in practice?

We might need to look at specific standards or substances to replicate the test.

Exactly! Standards like humic acids give us a good baseline to understand how our chemicals will behave in real-world scenarios. Remember, the context of your organic carbon source matters.
KOC versus KOW
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Moving on, let’s compare KOC with KOW. Can anyone tell me the main difference between these two coefficients?

KOW is about how chemicals partition in biological systems, right?

That's correct! While KOC focuses on organic carbon, KOW measure a chemical's affinity for lipid tissues, using octanol as a model for lipids. Why do you think this distinction is important?

It helps in toxicology and pharmacology to know how long a drug might stay in the body.

Spot on! Understanding both behaviors is essential for assessing both ecological impacts and therapeutic applications. High KOW can indicate high bioaccumulation potential.
Significance in Environmental Assessments
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let's tie this all together. Why is KOC and its understanding particularly relevant for environmental assessments?

It helps identify how contaminants can persist in the environment.

Correct! By analyzing KOC values, we can infer the potential for chemical contamination and remediation strategies. What are some pollution control measures that could be informed by KOC data?

We could focus on how to treat water or manage soil health to prevent contaminants from moving to groundwater.

Absolutely! KOC plays a vital role in shaping effective pollution control measures. It enables us to develop strategies that can mitigate environmental risks.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section explains the derivation and significance of the KOC value in understanding how different chemicals partition between organic carbon and water. It highlights the variability in organic carbon properties and the implications for environmental science and pharmacology, emphasizing the importance of KOC in assessing chemical behavior in natural systems.
Detailed
Detailed Summary
The relationship between a chemical’s partitioning behavior and organic carbon content is pivotal for understanding environmental dynamics.
-
KOC (Octanol-Water Partition Coefficient):
KOC represents the equilibrium between a chemical’s concentration in organic carbon compared to that in water. Specifically, it is defined as the ratio of the mass of a chemical (A) adsorbed onto organic carbon (OC) to the mass of that chemical in water. The expression is typically normalized, making it independent of the absolute concentration or the amount of OC, which allows for more generalized applications across different environments. -
Variability and Measurement:
Organic carbon varies based on its origin and composition; hence KOC values can differ significantly based on these factors. Experimental determination of KOC involves measuring the organic carbon content and deriving ratios using significant standards, such as humic and fulvic acids. -
Importance in Environmental Science:
KOC is crucial for evaluating how chemicals interact within soil and sediment systems, often acting as a natural purification medium. A high KOC suggests a tendency for the chemical to bind strongly to organic matter, which may lead to bioaccumulation in living organisms. Furthermore, KOC serves to predict chemical behavior in various ecosystems, including soil to groundwater interactions. -
Comparing KOC and KOW:
KOW (octanol-water partition coefficient) is similar but represents lipid partitioning in biological tissues as opposed to organic carbon. Understanding both KOC and KOW is essential for predicting ecological impacts and therapeutic efficacy in pharmacology, especially regarding how drugs partition in human tissues.
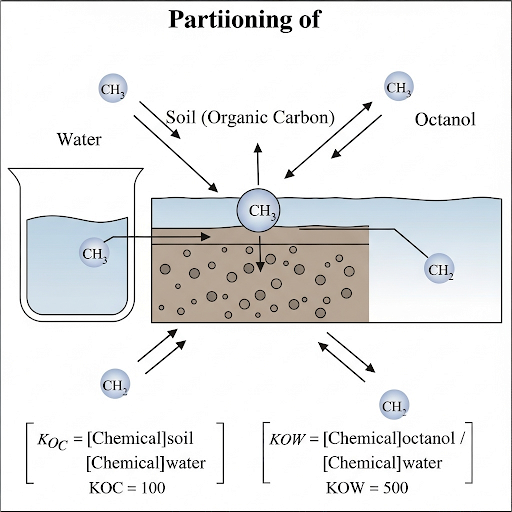
This chapter underscores the importance of these coefficients in environmental assessments and their implications in pollution management and substance exposure scenarios.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of KOC and Partitioning Concept
Chapter 1 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, this is we will use this in calculations, but this is not a, you can’t use it as a property of a chemical. But from this observation what people have looked at is that I can write this ‘KA32’ as ‘wA3’ just now we we have also determined the organic carbon content is different.
Detailed Explanation
This chunk introduces the concept of KOC (partitioning of a chemical between organic carbon and water) and explains how it relates to calculations in chemistry. Though KOC is not a chemical property in itself, it helps to analyze the behavior of chemicals in the environment. Specifically, it highlights that the observation of partitioning is influenced by the amount and type of organic carbon present.
Examples & Analogies
Think of KOC like a relationship between a variety of fruits (chemicals) and their affinity for a specific basket (organic carbon). Depending on the basket's structure (the type and amount of organic carbon), different fruits will choose to stay in that basket (partition) or drift away (dissolve in water). This metaphor helps to understand how KOC quantifies the tendency of a chemical to prefer either the organic phase (basket) or the aqueous phase (surrounding water).
Understanding Fractional Organic Carbon
Chapter 2 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Here, we are writing this as ‘wA3’. ‘wA3’ is m of A by ‘m’ of 3 right, if I write that in terms of this. So this first time here is the is the second term here is the content organic carbon containing it’s the amount of mass of carbon divided by mass of the solids. So we will give this, we call it as fractional organic carbon.
Detailed Explanation
This chunk discusses the concept of fractional organic carbon, which is an important factor in calculating KOC. It defines the ratio of mass of the chemical (A) to the mass of organic carbon (OC) present. This ratio indicates how much of the chemical is associated with the organic carbon relative to the total mass, playing a key role in understanding how chemicals partition between water and organic matter.
Examples & Analogies
Imagine you have a box of mixed candies (chemicals) and a small bowl of nuts (organic carbon). The fractional organic carbon is like saying, 'For every handful of nuts, how many candies do you have?' The more candies being added per handful, the stronger the preference (or ratio) for candies to stay with the nuts in that box, akin to how chemicals prefer organic carbon over water.
Normalization of Partition Constants
Chapter 3 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This number here becomes a little more normalised. So what it says is if I know what the organic carbon is in general I can now say that the adsorption of a particular chemical partitioning of a particle chemical on organic carbon with reference to water is should be more or less the same.
Detailed Explanation
This chunk explains the normalization of the partition constant, which is essential for comparing the behavior of different chemicals. By normalizing (i.e., adjusting for varying organic carbon content), KOC provides a consistent way to evaluate adsorption properties of chemicals on organic carbon, making it easier to compare results across studies and conditions.
Examples & Analogies
Consider a tasting competition among various chefs. If one chef makes a dish with a lot of salt and the other chef uses much less, you wouldn't compare their dishes directly based on taste alone. Instead, you'd normalize the salt content to understand their unique flavors better. Similarly, normalizing KOC helps contextualize how chemicals behave in varying environmental settings.
Variability of Organic Carbon
Chapter 4 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So here again we discussed in class that there is likely to be variability in organic carbon itself, but then, collection of a lot of data of people have figured out that the organic carbon has a certain range in which it behaves.
Detailed Explanation
The chunk addresses the variability in organic carbon across different settings, emphasizing that while this variability exists, research has established a certain behavioral range for organic carbon. Understanding this variability is crucial for accurate predictions and assessments of KOC, as it can influence how a chemical partitions in different environments.
Examples & Analogies
Imagine you are studying storm clouds. Some clouds are thick and heavy with water (high organic carbon), while others are wispy and light (low organic carbon). Although they may differ considerably in appearance and behavior, meteorologists have tools to gauge typical rain patterns. Similarly, researchers have methods to quantify how organic carbon behaves despite its variability.
Standardization of Organic Carbon Measurement
Chapter 5 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, when people measure organic KOC of a new chemical you need a reference right? Where do I get organic carbon from? I have to get it from I can get it from India, I can get from China, I can get it from the US, or Europe, Africa anywhere.
Detailed Explanation
This chunk emphasizes the importance of standardization in measuring KOC by procuring organic carbon from different regions around the world. The idea is that these references provide a baseline to which new chemicals’ KOC can be compared, ensuring a consistent and reliable approach towards understanding chemical behavior in environments.
Examples & Analogies
Think of a global cuisine competition where chefs from every continent bring their unique spices (organic carbon). To measure how one dish (chemical) stands out, judges must taste every dish with the same spices. This process ensures fairness in evaluating how well each dish integrates the spices used globally, just like using standardized organic carbon allows scientists to compare chemical behavior reliably.
Logarithmic Representation of KOC
Chapter 6 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, this log and usually represented as log KOC, because KOC is a big number and you can imagine even if I have a few milligrams of organic carbon, solubilitys of a lot of these chemicals is small, but they have organic they have, they like they like organic phase.
Detailed Explanation
This chunk explains the logarithmic representation of KOC, which helps simplify the large numbers typically associated with KOC values for various chemicals. By using logarithms, scientists can more easily communicate and compare the affinity of chemicals to bind to organic phases versus being in water.
Examples & Analogies
Using logs to represent KOC is like summarizing a long book into a catchy headline; the headline captures the book's essence and makes it easier to communicate. Instead of remembering complex details, a simple number becomes shorthand that conveys complex information about a chemical's behavior.
Practical Implications of KOC
Chapter 7 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So this hydrophobic behavior gives you a quick reference for remediation or treatment or whatever that you need to do subsequent decision to it.
Detailed Explanation
This chunk highlights the practical implications of understanding KOC and its association with hydrophobicity. By knowing how different chemicals interact with both water and organic carbon, scientists and environmentalists can make informed decisions about remediation efforts and environmental management.
Examples & Analogies
If you're trying to clean up an oil spill (hydrophobic materials) in a lake (water environment), knowing whether a chemical is more likely to cling to the oil or disperse in water helps guide your cleanup strategy. Understanding KOC helps prioritize efforts based on the chemical’s behaviors, leading to more effective solutions.
Key Concepts
-
KOC: Indicates the ratio of concentration of a chemical in organic carbon to that in water, used to evaluate environmental behavior.
-
KOW: A similar concept, but evaluates distribution in biological systems using octanol as a proxy for lipids.
-
Organic Carbon Importance: Source and composition significantly affect KOC values and thus chemical partitioning and behavior.
-
Adsorption: The mechanism by which chemicals adhere to organic carbon, impacting their environmental fate.
Examples & Applications
Example 1: The assessment of a pesticide's KOC could inform if it will persist in soils versus leaching into groundwater, impacting remediation strategies.
Example 2: Understanding the KOW of a pharmaceutical helps predict its bioaccumulation potential and efficacy in human tissues.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
For KOC to show, in soil it will flow, to water it won't go, strong binding is the show.
Stories
Imagine a scientist in a lab, measuring the KOC of socks soaked in water, learning how they grab onto dirt and pollutants without letting go, showcasing the power of organic matter.
Memory Tools
KOC: Keep On Carbon - remember that KOC originates from interactions with organic carbon.
Acronyms
KOC
Knowledge Of Carbon - emphasizing the need for understanding organic sources in chemical evaluations.
Flash Cards
Glossary
- KOC
The partitioning coefficient that indicates the ratio of a chemical's concentration in organic carbon to its concentration in water.
- KOW
The octanol-water partition coefficient, comparing a chemical's distribution between octanol and water, which serves as a surrogate for lipids.
- Organic Carbon
A component of soils and sediments largely derived from decayed plant and animal matter used in assessing KOC.
- Adsorption
The process by which atoms, ions, or molecules from a gas, liquid, or dissolved solid adhere to a surface.
- Bioaccumulation
The accumulation of substances, such as pesticides or other chemicals, in an organism.
Reference links
Supplementary resources to enhance your learning experience.
