Identification and Monitoring of Hazardous Chemicals
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Pollutants and Classification
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will discuss what constitutes a pollutant. Essentially, any substance that can cause harm to the environment or human health can be classified as a pollutant. Can anyone think of how we could classify these pollutants?

Could we categorize them as toxic and non-toxic?

That's a great start! Now, let’s discuss more foundational classifications. In chemistry, how do we typically categorize chemicals?

Into organic and inorganic categories?

Exactly! So, let’s break down organic chemicals. Organic chemicals include aliphatic and aromatic compounds. Can anyone give me an example of an aliphatic compound?

How about methane?

Yes! Methane is a simple aliphatic compound. It's crucial to understand these classifications since many pollutants arise from organic compounds. Now, why do we need to monitor these chemicals?

To protect human health and the environment!

Correct! Monitoring helps us understand potential risks. Let’s summarize: pollutants can be classified in various ways, with organic compounds being significant. Monitoring these chemicals is essential for our safety.
Organic and Inorganic Chemicals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

In our last session, we touched on organic chemicals. Now, let’s explore inorganic chemicals. Who can tell me what makes a chemical inorganic?

I think they don't contain carbon?

Exactly! Common examples include heavy metals, which are critical in our discussion of pollutants. Can anyone name some hazardous heavy metals?

Lead and mercury?

Great examples! Both have severe health implications. Transitioning to organic chemicals again, can anyone explain why substances like PCBs and PAHs are recognized as chemicals of concern?

Because they are toxic and can persist in the environment?

Exactly! This persistence contributes to their hazardous nature. Now, let’s summarize: organic compounds include aliphatic and aromatic types, whereas inorganic chemicals often consist of heavy metals that pose risks.
Applications of Chemicals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

We've learned about the classifications of chemicals. Let’s move on to their applications. How do you think the classification impacts their use in industries?

Different industries would need specific types based on required properties?

Correct! For example, in agriculture, we use fertilizers and pesticides—both chemical applications. What about in construction?

Paints and solvents are commonly used there.

Right! Solvents help in creating paint solutions. These applications demonstrate how essential chemical monitoring is to prevent adverse health effects from hazardous substances. Can anyone summarize our discussions on applications and their impact?

The classification of chemicals dictates their application in various industries, which necessitates the monitoring for health safety.

Well-said! Monitoring certain chemicals is vital. As we conclude this session, remember the critical link between chemicals, applications, and monitoring.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section elaborates on different classifications of hazardous chemicals, including organic and inorganic compounds. Key points include the importance of categorizing pollutants based on their properties and applications, with an emphasis on specific examples like chlorinated compounds and polyaromatic hydrocarbons. The significance of monitoring these substances to ensure environmental safety is also highlighted.
Detailed
Detailed Summary
In this section, we dive into the classification of hazardous chemicals and the importance of monitoring them to safeguard human health and the environment. The main classifications emerge from a toxicological perspective, wherein a chemical, termed as 'A', can either be toxic or non-toxic. The fundamental division of substances is rooted in their basic properties, leading us to a deeper classification as organic and inorganic chemicals.
Organic Chemicals
Organic chemicals can be broadly classified further into:
- Aliphatic Compounds: These include substances such as alkanes, alkenes, and alkynes. For example, methane (CH₄) and dichloromethane (CH₂Cl₂) illustrate the variety within this category.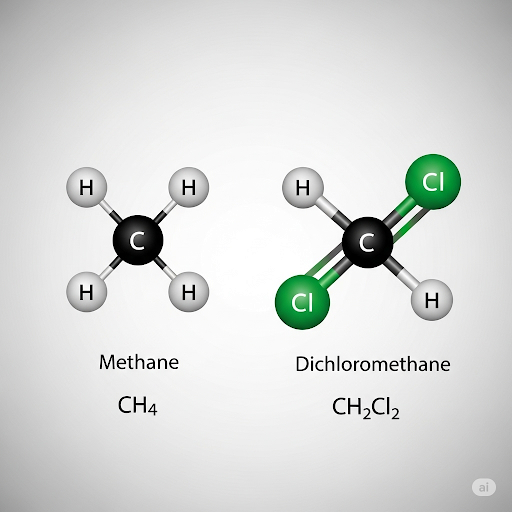
- Aromatic Compounds: Defined by the presence of benzene rings, compounds like benzene itself and its derivatives, including polychlorinated biphenyls (PCBs) and polyaromatic hydrocarbons (PAHs), fall into this group. They are significant due to their widespread use and potential toxicity.
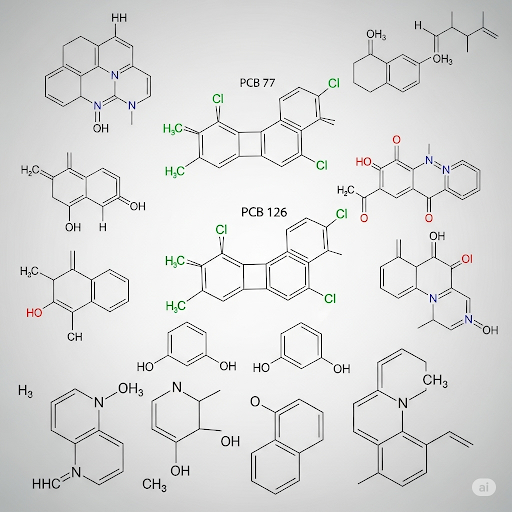
Inorganic Chemicals
On the inorganic side, the discussion includes various heavy metals (like lead and mercury) and their implications as environmental pollutants. These compounds often manifest in elemental forms or combined as salts or oxides, making their presence in the environment complex and challenging to monitor.
The lecture also indicates the practical importance of these classifications with respect to their applications across industries. Chemicals become 'chemicals of concern' when evidence suggests they may cause health issues. Industries utilize these chemical classifications in their operational processes, including in agriculture (fertilizers and pesticides), industrial manufacturing (solvents and paints), and other chemical applications. This understanding further emphasizes the need for effective monitoring to prioritize which chemicals to track based on their potential health impacts. Through a structured approach to classification, monitoring those deemed hazardous becomes manageable, ensuring environmental quality and safety.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Identification of Chemicals of Concern
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, these are chemicals of concern. So, anything can become a chemical of concern if there is evidence to believe that it can cause human health problems and then we need to track this.
Detailed Explanation
In this chunk, we introduce the concept of 'chemicals of concern.' A chemical becomes classified as a chemical of concern when there is scientific evidence suggesting it may pose a risk to human health. This could include compounds found in everyday products, pollutants in the environment, or substances used in industries. The identification process is essential because it directs attention and resources towards monitoring these chemicals to minimize any potential harm to people and the environment.
Examples & Analogies
Imagine a community where people are getting sick, and doctors suspect that it might be related to a chemical used in agriculture nearby. Investigating this chemical is similar to a doctor figuring out what makes a patient ill. They analyze what the patients have been exposed to and determine if that chemical could cause their symptoms.
Application-Based Classification
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This is based on the structure, chemical structure and composition, but application based from a utility point of view, you can also classify the chemicals of your interest or chemicals of concern.
Detailed Explanation
This chunk explains how we not only categorize chemicals based on their chemical structure but also consider how they are used in various applications—this is called application-based classification. For example, we can classify chemicals used in agriculture, such as pesticides and fertilizers, as those that concern us in the context of food safety and environmental health. By looking at where and how chemicals are applied, we can focus our monitoring efforts on the most relevant substances.
Examples & Analogies
Think of a chef who organizes ingredients in their kitchen. Instead of sorting everything by color or size, they group items together by how they're used—such as spices, oils, and vegetables. Similarly, researchers categorize chemicals not just by what they are, but by what they do, which helps in understanding the potential risks associated with their usage.
Real-World Applications of Hazardous Chemicals
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, when we categorize chemicals based on application, for example, we could mention disinfectants, fertilizers, pesticides, and paints. These substances, although beneficial in their respective applications, can pose health risks if not managed properly.
Detailed Explanation
Here, we delve into the different applications of hazardous chemicals. Common examples include disinfectants used for cleaning and sanitizing surfaces, fertilizers applied to enhance crop growth, and pesticides used to control pests in agriculture. While these chemicals can have positive effects, such as improving hygiene or agricultural productivity, they also carry inherent risks, particularly if exposure is not controlled or if there are accidents in their application. This highlights the need for careful monitoring and management strategies.
Examples & Analogies
Consider a gardener who uses fertilizers to grow plants more effectively. While the plants flourish, if the gardener isn't careful about how much fertilizer they use, it could run off into local water supplies, leading to harmful pollution. This showcases the dual nature of chemicals as both beneficial and potentially hazardous.
Importance of Monitoring Hazardous Chemicals
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
is there a way in which I can, then it becomes a matter of engineering decision making that I have to spend my money and resources and time where I can guess whether one of these is likely to be there.
Detailed Explanation
This chunk emphasizes the complexity and importance of monitoring hazardous chemicals. Due to the vast number of chemicals that could potentially be present in any given environment, it is critical to prioritize which substances to monitor based on likelihood and potential risk. This decision-making process involves balancing costs, available resources, and the need for accurate assessments to safeguard health and the environment.
Examples & Analogies
Think about a detective trying to solve a case. Instead of looking into every possible suspect, they focus on the ones who have a motive and opportunity. Similarly, environmental scientists need to focus their investigations on the most likely hazardous chemicals that could harm health, rather than attempting to analyze everything at once, which would be inefficient and costly.
Key Concepts
-
Pollutants: Any substance that can harm health or the environment.
-
Organic vs. Inorganic Chemicals: Understanding the fundamental difference in chemical categorization.
-
Aliphatic Compounds: A class of organic chemicals characterized by carbon chains.
-
Aromatic Compounds: Organic chemicals containing benzene structures, potentially hazardous.
-
PCBs and PAHs: Specific examples of chemicals of concern.
Examples & Applications
Methane (CH₄): An example of an aliphatic compound.
Benzene (C₆H₆): A common aromatic compound that is hazardous.
Mercury (Hg): A toxic heavy metal that is prevalent in environmental discussions.
PCBs: Industrial chemicals that have significant environmental persistence and toxicity.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In the ocean, heavy metals swim, lead and mercury - they cause a grim.
Stories
Once, there was a little factory by the river, releasing paints and solvents that made the fish shiver. But monitoring the chemicals helped keep pollution at bay, ensuring the health of creatures every day.
Memory Tools
Remember the 'PAL' of hazardous chemicals: Pollutants Are Lethal!
Acronyms
PCBs
Persistently Causing Biotoxicity.
Flash Cards
Glossary
- Pollutant
A substance that can harm the environment or human health.
- Organic Chemicals
Compounds mainly consisting of carbon, including various subcategories.
- Inorganic Chemicals
Substances that do not typically include carbon.
- Aliphatic Compounds
Organic compounds composed of straight or branched chains of carbon.
- Aromatic Compounds
Organic compounds containing benzene rings.
- Heavy Metals
Metal elements with a high atomic weight that can be toxic.
- PCBs (Polychlorinated Biphenyls)
Industrial chemicals that are hazardous and persistent in the environment.
- PAHs (Polyaromatic Hydrocarbons)
Organic compounds with multiple aromatic rings, often harmful to health.
Reference links
Supplementary resources to enhance your learning experience.
