Partition Constants for Other Systems
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Soil Saturation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are going to explore how water content in soil influences its interactions with chemicals. Can anyone explain what we mean by saturated and unsaturated soil?

Saturated soil is when all the pores are filled with water, right?

Exactly! And unsaturated soil contains both air and water in its pores. This is where we describe the moisture status using terms like wet, damp, and dry. Can anyone summarize these terms?

Wet means there’s full coverage with water, damp has less than that, and dry means no water at all.

Great summary! Remember, these classifications are essential for understanding how chemicals partition in soils.
Chemical Interactions in Unsaturated Soil
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand moisture levels, let’s talk about how these levels impact chemical interactions. What do you think happens to a chemical when soil is wet?

If the soil is wet, the chemical binds to organic carbon instead of minerals.

Excellent point! And in damp soil, the chemical can access both organic carbon and some mineral surfaces. What about dry soil?

In dry soil, the chemical can bind to organics and minerals, making it more accessible.

Correct! So, understanding the moisture content is crucial for predicting how a chemical will behave in the soil.
Importance of Partition Constants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s move on to partition constants, specifically KA31. Why do you think knowing KA31 is important?

It helps us understand how chemicals move and react within soil.

Exactly! KA31 varies depending on moisture content. In what scenario do you think KA31 would be highest?

In dry conditions, because more surfaces are available for chemical interaction.

Right! This knowledge is vital for environmental modeling and predicting contamination behavior.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, we explore the concept of partitioning in unsaturated solid systems such as soil. It delineates how moisture conditions like wet, damp, and dry affect chemical interactions with soil, emphasizing the significance of moisture content in defining partition constants.
Detailed
Detailed Summary
This section delves into partition constants relevant to unsaturated solid systems, primarily focusing on soil as a key example. It outlines the differences between saturated and unsaturated zones within soil, explaining that unsaturated soil contains a mix of water and air in its pore space. The concepts of wet, damp, and dry soil are elaborated upon, where 'wet' indicates full monolayer coverage by water, 'damp' less than one monolayer, and 'dry' signifying no water present.
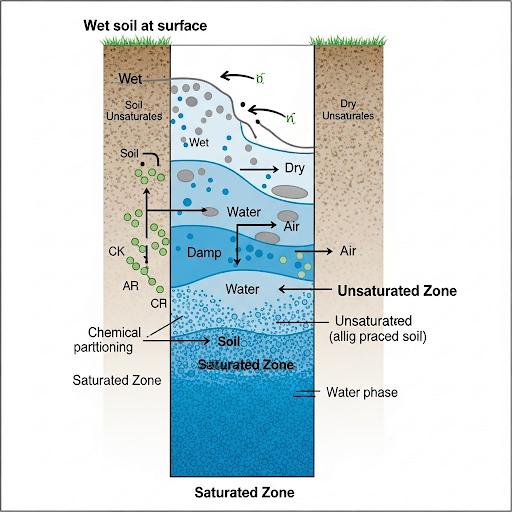
The implications of these moisture states on chemical partitioning are discussed, highlighting how chemicals can interact with organic carbon or mineral surfaces depending on the moisture content. The partition constant KA31 is introduced, indicating its dependence on moisture conditions. The section also emphasizes that knowledge of moisture content is crucial for predicting partitioning behavior and the equilibrium between air, water, and organic carbon within soil systems. This understanding of partition constants is vital for modeling chemical transport processes in the environment.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Soil Moisture States
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Soil can be classified into three moisture states: wet, damp, and dry.
1. Wet: This state indicates complete monolayer coverage of water on the mineral surface.
2. Damp: This state means there is less than one monolayer coverage of water, with regions having water and others not.
3. Dry: This indicates there is no significant water on the mineral surfaces.
Detailed Explanation
Soil moisture greatly influences its physical and chemical properties. In a wet state, soil is saturated with water, meaning all surface areas of the mineral particles are covered with at least one layer of water. In the damp state, though water is present, it does not cover the full surfaces completely; thus, some mineral areas are still exposed. In contrast, dry soil contains very little to no water at all. Understanding these states is crucial for predicting how conditions affect soil interactions, such as chemical partitioning.
Examples & Analogies
Think of a sponge: when it is fully soaked, it's like 'wet' soil, where every pore is filled. When it’s partly filled, some areas are still dry, similar to 'damp' soil. Finally, when the sponge is left out and no water is inside, it represents 'dry' soil.
Chemical Behavior in Different Soil Moisture States
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The behavior of chemicals in soils varies based on moisture content.
- In wet soils, chemicals bind primarily to organic carbon because there's no competition from water.
- In damp soils, chemicals can bind to both organic carbon and mineral surfaces.
- In dry soils, they can accumulate on both organic matter and mineral surfaces.
Detailed Explanation
The way chemicals interact with soil constituents is highly dependent on moisture levels. In wet soil, the presence of water prevents chemicals from interacting with mineral particles, directing their binding mainly to organic carbon. In damp conditions, however, there's room for interaction with both organic materials and exposed mineral sites. Dry conditions create an environment where chemicals can readily bind to both, as there’s no water inhibiting access. This is important for understanding soil contamination and nutrient availability.
Examples & Analogies
Imagine pouring syrup over different kinds of bread. Over a whole loaf (wet), the syrup may only stick to spots with butter (organic carbon). On a piece that’s half toasted (damp), it can mix with both the butter and dryer parts. In a completely stale piece of bread (dry), the syrup can coat the entire surface.
Partitioning and Soil Moisture Content
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The partitioning of chemicals is influenced by soil moisture content:
- As moisture content changes, the value of the partitioning coefficient (KA) varies.
- The order of KA values is dry > damp > wet, meaning drier soils lead to greater chemical partitioning.
Detailed Explanation
Partitioning refers to the distribution of a chemical between different phases—in this case, between soil components and water or air. As moisture content decreases, the prevalence of chemicals binding to mineral surfaces increases because less pore space is filled with water. Thus, drier soils tend to show higher partitioning coefficients compared to damp or wet soils, which can be critical in environmental assessments and soil remediation efforts.
Examples & Analogies
It’s like how a sponge absorbs water: the drier the sponge, the more water it can hold when you dip it in. If saturated, it cannot hold more; similarly, in dry soils, chemicals find ample space to bind.
Measuring Partition Constants
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The measurement of partition constants involves achieving equilibrium between air and water phases by introducing a chemical and allowing it to stabilize at different conditions.
Detailed Explanation
To measure partition constants effectively, a careful setup must ensure that the chemical can reach a state where its amount remains stable across phases. By measuring concentrations at this equilibrium, calculations can yield valuable constants that describe how much of a chemical is likely to be found in one phase versus another. This process includes ensuring that the system is closed and that no external factors alter concentration. Sometimes, trials help determine the necessary time for equilibrium, making experimental design scalable.
Examples & Analogies
Think of preparing a drink mix. If you dump in the powder and wait, you'll notice that after some time, no more will dissolve into water—it’s reached its limit, just like reaching equilibrium in partition measurement.
Key Concepts
-
Unsaturated vs. Saturated Soil: Unsaturated soil contains water and air while saturated is fully filled with water.
-
Partitioning Behavior: The interaction of chemicals with soil depends on moisture content.
-
Moisture Levels: Wet, damp, and dry classifications impact how chemicals bind to soil components.
Examples & Applications
After a rainfall, the top layer of soil may feel wet, but when digged, you might not see free water as it is retained in thin films around soil particles.
In dry soil, herbicides can bind more efficiently to mineral surfaces compared to when the soil is wet.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Wet, damp, dry – soil's moisture types; know them well to avoid big gripes!
Stories
Imagine walking in the rain. You find soil that's wet, damp, and dry. Each condition tells a unique story of water's journey through soil.
Memory Tools
WDD - Wet has Diverse coverage; Damp is Deficient; Dry has none.
Acronyms
WDD for Wet, Damp, Dry classifications.
Flash Cards
Glossary
- Unsaturated Soil
Soil that contains both air and water in the pore spaces.
- Saturated Soil
Soil where all the pore spaces are filled with water.
- Partition Constant (KA31)
A measure of how a chemical partitions between organic carbon and soil water.
- Monolayer Coverage
A surface covered by a single layer of water molecules.
- Moisture Content
The amount of water present in the soil, affecting its physical and chemical properties.
Reference links
Supplementary resources to enhance your learning experience.
