Discussion on Interferences and Filtration
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Extraction Techniques
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to learn about extraction methods for analyzing organic compounds in water. Can anyone tell me why extraction is necessary?

Is it to separate the compounds from the water?

Exactly! We need to separate these compounds as they exist in very low concentrations, often in the nanogram or microgram per liter range. What kinds of solvents do you think we might use for this process?

Could we use something like hexane?

Correct! Hexane and dichloromethane are commonly used because they are good at dissolving organic compounds. However, remember that these solvents must be immiscible with water. Let's add a memory aid: you can think of HEXANE as 'Helping Extracting eXtra Active Natural Elements!' This can help remember its purpose in extraction.

What do we do about the hazardous nature of these solvents?

Excellent question! Waste management is crucial. Always ensure proper disposal of hazardous solvents to prevent environmental contamination.

So, we have to consider the extraction method carefully?

Absolutely! The extraction method must be selected based on the analyte and the sample's matrix. Let’s summarize: extraction is essential for isolating compounds and must be done using appropriate, safe solvents.
Identifying and Managing Interferences
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss interferences. Can someone explain what interference means in analytical chemistry?

Isn't it any substance that affects the results of our analysis?

Exactly! In the context of our water samples, if we have, say, PAHs and oils, what might happen if we try to analyze just the PAHs?

The oil could interfere with our measurement of PAHs.

Right! The key take-home point is that interferences are specific to the analytes of interest. It's important to recognize these during sample collection and analysis. Can anyone give me an example of how to reduce interferences?

We should filter the samples before extraction?

That's correct! Filtration is the first step to minimize interferences. Well done! Let's recap what interferences are and emphasize their relative nature to the analyte being studied.
Filtration Techniques and TSS
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Filtration plays a crucial role in preparing our samples. Can someone explain what we measure through filtration?

We measure total suspended solids, right?

Yes! TSS is essential for understanding the sample's composition. What kind of filters do we need to use here?

Filter papers have different pore sizes. We can choose based on what we want to remove.

Exactly! But remember, using a filter with a very small pore size can cause issues. What might happen?

It might take too long to filter, or it could clog up.

Right again! That's why a balance, like the 1-micron filter, is often used—it allows enough flow while still filtering effectively. Let's summarize: filtration is key for determining TSS, and choosing the right filter is essential for efficiency and accuracy.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section provides an in-depth look at the processes of extraction and filtration in water analysis. It explains the principles behind liquid-liquid extraction, the role of solvents, potential interferences that can affect analytical results, and the significance of using appropriate filtration techniques to ensure accurate data.
Detailed
Detailed Summary
In this section, we explore the critical steps in analyzing organic compounds in water, focusing on extraction and filtration methods. Extraction is particularly essential when working with low concentrations of organic compounds, typically measured in nanograms or micrograms per liter. Using compatible solvents, we can perform liquid-liquid extraction, where organic compounds are transferred from a water matrix to an immiscible solvent. Common solvents like hexane and dichloromethane are highlighted for their effectiveness, though their hazardous nature necessitates careful waste management.
One significant challenge in analyzing organic compounds arises from the presence of interferences, particularly when samples originate from untreated water sources, such as wastewater treatment plants. Here, other organic materials can co-extract alongside target analytes, leading to potential inaccuracies in quantifying specific compounds. Therefore, understanding what constitutes interference is essential—interference is defined relatively, based on the analyte of interest.
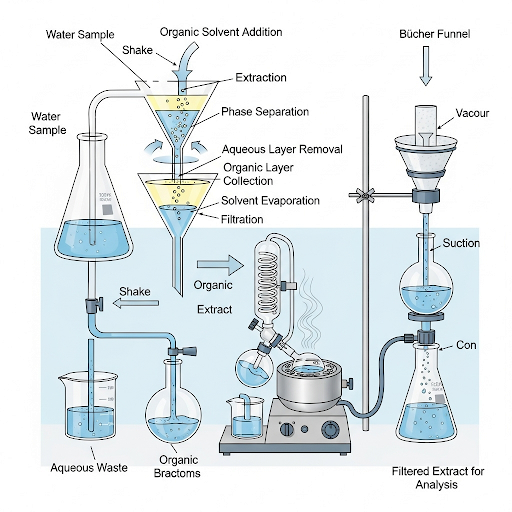
Filtration is a crucial preparatory step to minimize these interferences. By measuring total suspended solids (TSS) through filtration, one can separate solids from the water sample before extraction. Various types of filter papers are available, each defined by pore size, and the choice of filter can significantly influence the analysis success. The section outlines the importance of selecting the appropriate filter paper and discusses the trade-offs between pore size and filtration efficiency. Ultimately, this comprehensive approach to extraction and filtration in water quality analysis ensures more reliable and accurate results.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Interferences in Analysis
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When you extract water as it is raw water, sometimes you will get everything, all the interferences in your sample... These are interferences. So I must do something with these 2 before I go on the analysis of PAHs.
Detailed Explanation
In the analysis of water samples, especially those from wastewater or sewage treatment, it is crucial to recognize that other materials present in the sample can interfere with the target analytes. For example, if we are analyzing for polycyclic aromatic hydrocarbons (PAHs), the presence of oils or metals in the water could skew our results. Thus, we need to identify these substances and address them to ensure accurate measurements.
Examples & Analogies
Think of it like trying to taste soup when there are too many spices in it. If you’re trying to identify the flavor of basil but there’s too much garlic, it will be hard to pinpoint the basil flavor. Similarly, interferences in water samples can overshadow the analytes we want to measure.
The Need for Filtration
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, before this you have to do liquid-liquid extraction, if you don’t want that information, you have to filter the samples... Normal sequence of events that you will see is the sample is collected then we move on, after this we do the next step.
Detailed Explanation
Filtration is a necessary step before conducting liquid-liquid extraction as it helps remove larger particles that could interfere with the analysis. By filtering the sample first, we ensure that only the dissolved substances remain, allowing for more accurate assessments of what we wish to analyze in the water sample.
Examples & Analogies
Imagine you are preparing to bake a cake. If you have flour mixed with big lumps, you wouldn't want to include those lumps in your mix. So, you sift the flour first to get a smooth mixture. Similarly, filtering the water samples gives a clear solution to analyze.
Total Suspended Solids (TSS)
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When you say total suspended solids it is we are taking a water sample and filtering it through a filter paper... mass of filter that is collected here divided by the volume of water will give you total suspended solids.
Detailed Explanation
Total suspended solids (TSS) refer to the amount of particulate matter present in the water that can be captured on a filter. After filtering a water sample, the mass of the solids collected on the filter paper is divided by the volume of water filtered to determine the concentration of TSS. This measurement is important in assessing water quality, particularly for environmental monitoring.
Examples & Analogies
Think of TSS like the residue left in a coffee filter after brewing coffee. The grounds that remain after filtering represent the solid particles (suspended solids) that can affect the clarity and quality of your drink (or in this case, the water body).
Choosing the Right Filter Paper
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
There is one piece of information that is needed here is, what is the filter paper that we use?... So, it will say 10 microns or it will say 1 micron you can go up to 0.4 microns.
Detailed Explanation
The choice of filter paper is crucial because different filters have varying pore sizes that determine what can pass through them. Using too fine a filter can lead to longer filtration times and potential clogging, while too coarse a filter may allow unwanted particles to pass through. The right balance is key for effective filtration.
Examples & Analogies
Selecting the proper filter is akin to choosing the right strainer for cooking pasta. If your strainer has too large holes, you'll end up with pasta in your sink. If it’s too small, water won't strain easily. Thus, a balance between size and functionality is necessary.
Understanding Filtration Efficiency
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If I take a filter say I use 1 micron glass fiber filters... 0.7 is not really needed and 1 micron is probably good enough to do whatever collide removal you want.
Detailed Explanation
Using different pore sizes of filters can significantly affect the efficiency of filtration and the purity of results. While a more precise filter may seem beneficial, it can slow down the process and provide negligible benefits in terms of the additional small particles removed. Thus, a 1 micron filter is often deemed sufficient for most analysis purposes.
Examples & Analogies
If you think of filtering while making homemade lemonade, using a fine cheesecloth to strain out pulp may seem like a good idea, but it takes so long to pour. Using a coarser strainer can speed up the process without sacrificing much taste or quality.
Standardizing TSS Measurement
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, 1 micron is set as standard filter size for TSS because it is TSS standard filter size... but still there is this question one has to draw a line somewhere.
Detailed Explanation
1 micron filters are generally accepted as the standard for measuring total suspended solids as they provide a reliable method for quantifying solid content in samples without introducing excessive filtration time or complexity. It’s a practical choice in balancing precision and efficiency when analyzing water quality.
Examples & Analogies
Much like how standard measurements like a tablespoon or cup are used in cooking to ensure consistent results, using a 1 micron filter in TSS measuring ensures that everyone gets reliable results, making comparisons across samples easier.
Key Concepts
-
Extraction: The process of isolating desired compounds from a liquid mixture using solvents.
-
Interference: Substances in a sample that may alter the analytical results.
-
Filtration: A method for separating solids from liquids to prepare samples for analysis.
Examples & Applications
Using hexane as a solvent to extract organic compounds from water.
Analyzing a wastewater sample for PAHs, where oils and solids might interfere.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Extraction's a reaction, solvents in action, pulling out compounds, for data extraction.
Stories
Imagine a scientist on a quest, seeking compounds hidden in water's breast. With hexane in hand, they filter away to unveil what lies beneath the spray.
Memory Tools
Remember 'I Filter Before I Extract' to remind you to always filter your samples first.
Acronyms
USE - Understand Solvent Extraction
guide to remember your solvent choices.
Flash Cards
Glossary
- Extraction
A process used to separate specific compounds from a mixture, typically using a solvent.
- Interference
Any substance in a sample that can affect the measurement of the target analyte.
- Dichloromethane
A chlorinated solvent commonly used in organic extractions, known for its ability to dissolve a wide range of compounds.
- Total Suspended Solids (TSS)
A measure of the solids present in water that can be trapped by a filter.
- Filtration
The process of separating solids from liquids using a porous material.
Reference links
Supplementary resources to enhance your learning experience.
