Mass Balance Analysis
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Mass Balance
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's start with the fundamentals of mass balance. What do you think mass balance means in an environmental context?

I believe mass balance is about accounting for all the mass in a system.

Exactly! We track the mass before and after a process to ensure nothing is lost. Can anyone provide an example?

When we add water to a sludge tank, we need to account for how that changes the mass of solids and liquids.

Perfect! This analysis is crucial for industries managing waste. Let’s see how we apply it to a real-world scenario in our next discussion.
Analyzing the Waste Tank Scenario
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Imagine a waste tank with 600,000 kg of wet sludge. What do we know about this sludge?

It has a moisture content of 40%, meaning 40% of the mass is water.

And it contains 15% organic carbon on a dry basis!

Spot on! Now, when rainwater mixes in, we say it creates a suspension. How does that alter our mass balance?

The rainwater adds volume and might dilute the concentration of chemical A in the sludge.

Exactly! Understanding these changes is key. Let’s move on to setting up a mass balance equation based on these observations.
Equilibrium and Assumptions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

What must we assume about the system when we analyze it at equilibrium?

That there are no losses from evaporation or other reactions happening.

Yes! This simplifies our calculations significantly. If we assume no additional A is introduced, what does that lead us to conclude?

It means we can focus solely on how mass redistributes among the solid and liquid phases.

Right! So the overall balance remains intact, and we can predict concentrations after mixing.
Calculating Concentrations
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s calculate the expected concentration of chemical A in the water after it's mixed. What relationship do we use?

We set the mass of A in the sludge equal to the mass of A in water after mix.

Exactly! By calculating the mass of A in both phases, we can find the concentration in each, right?

Yes! And we also need the volumes to get the final concentration values.

Great work! As we proceed, keep an eye on how volumes change with mixing, as this significantly affects our calculations.
Real World Application and Implications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Why do you all think understanding mass balance is crucial in environmental engineering?

It’s important for ensuring pollutants are tracked and managed effectively.

And it helps in designing better waste management strategies.

Absolutely! Accurate mass balance contributes to effective pollution control and understanding environmental impacts.

I can see how this is vital for industries and environmental protection efforts.

Exactly, great insights everyone. Keep these discussions in mind as we move forward into more complex scenarios.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section explores the concept of mass balance within an industrial waste tank, highlighting the processes involved in mixing, settling, and analyzing concentrations of chemical components after the introduction of rainwater. It emphasizes the importance of understanding equilibrium conditions and the assumptions necessary for accurate mass balance calculations.
Detailed
Mass Balance Analysis
In this section, we focus on mass balance analysis exemplified by an industrial waste tank scenario. The analysis starts with a waste tank that contains solid sludge, which is a mixture of water and solids. Key parameters given are the moisture content of the sludge, the percentage of organic carbon, and the loading of a specific chemical (referred to as chemical A). Initially, the sludge occupies a height of 1 meter in the tank, and a total mass of wet sludge is established.
Overview of the Mass Balance Procedure
When rainwater is introduced into the tank, it mixes with the existing sludge, transforming it into a slurry that occupies a total height of 3 meters. This mixing leads to redistribution of chemical A between the solid phase (sludge) and the aqueous phase (water). The process proceeds through various stages:
1. Initial State (Stage 1): The sludge is uniformly mixed, containing a specific concentration of chemical A.
2. Mixing Event (Stage 2): Upon adding rainwater, the sludge forms a suspension, allowing for interactions between the solid and liquid components.
3. Equilibrium State (Stage 3): After settling occurs, the sludge retains some chemical A while some of it disperses into the water.
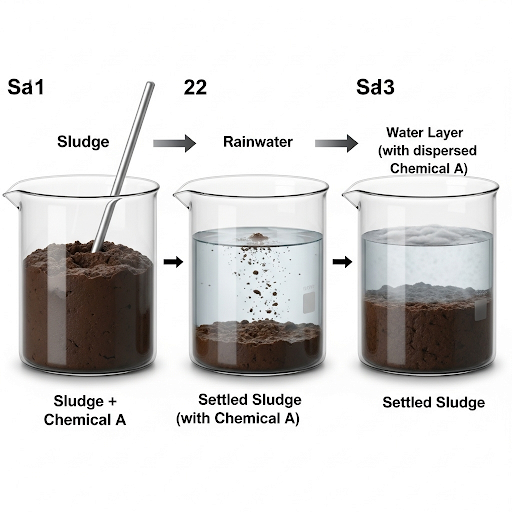
Performing Mass Balance Calculations
The mass balance can be summarized in the equation:
Mass of A in sludge before equilibrium = Mass of A in water after equilibrium
We assume that the rainwater does not introduce any additional mass of chemical A (i.e., it contains no A), a simplification that allows for clearer calculations.
Key Assumptions and Equilibriums
- No evaporation occurs during the process.
- The concentration of A can be calculated based on the mass of solids and the respective volumes involved.
- Organic carbon's presence impacts the concentration calculations but is treated as a constant during this analysis.
Understanding the dynamics of mass balance is pivotal for environmental monitoring and industrial processes, ensuring the accurate assessment of contaminant levels in water and sludge systems. This analysis showcases the foundational principles of environmental engineering that inform effective waste management strategies.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to the Problem Statement
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
An industrial facility has a waste tank containing a small amount of solid sludge, which is water plus solids consisting of inorganic and organic components. The sludge occupies about 1 meter of the tank uniformly spread across. The moisture content of the sludge is 0.4, and the percentage of organic carbon in the sludge is estimated as 15% dry basis.
Detailed Explanation
In this part, we are introduced to a waste tank in an industrial facility filled with sludge. The sludge is a mixture of water and solids, which can also include harmful chemicals. The moisture content and organic carbon percentage are critical for calculating the mass balance. The moisture content indicates that a certain percentage of the sludge is water. The organic carbon content is important for understanding the environmental impact of the waste.
Examples & Analogies
Imagine you have a large bowl filled with mud (the sludge). The mud is made of sand (solids) and water. Just like how the amount of sand and water influences how the mud behaves, in our waste tank, the moisture level and the amount of organic carbon determine how the sludge will interact with other chemicals and how it will affect the environment.
Changes Due to Rainwater Addition
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
During a rain event, rainwater mixes with the sludge, converting it into a slurry. After mixing, the entire content occupies 3 meters of height in the tank—2 additional meters of water added from the rain.
Detailed Explanation
When rainwater is added to the sludge, it dilutes the contents, turning the mixture into a slurry. This increases the height of the material in the tank because the rainwater raises the total volume from the initial 1 meter to 3 meters. The mixing process helps distribute the chemicals even more thoroughly throughout the water and solids.
Examples & Analogies
Think of adding water to a sandy beach. If you pour enough water on sand, it will become muddy and the overall volume will increase, similar to how the rainwater increases the height in our tank. The contents mix, and just like how water can carry more sand when it’s mixed, rainwater carries the contaminants present in the sludge.
Understanding Mass Balance
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For this setup, we need to analyze what happens to the chemical A concentrated in the sludge. The crucial question is to find out the concentration of A in the aqueous phase after the water is drained off for analysis.
Detailed Explanation
We are tasked with calculating the concentration of chemical A in the water after it has been mixed with the sludge. This involves applying the principle of mass balance: the mass of A in the sludge before mixing should equal the mass of A in both the remaining sludge and the water after mixing. By setting up the mass balance equations correctly, we can determine how much of chemical A has transferred to the water.
Examples & Analogies
Imagine you have a sponge soaked in colored water (the sludge). When you squeeze it (drain off the water), some colored water comes out while some stays in the sponge. Analyzing how much color is in the water can tell you how much was originally in the sponge. This mass balance helps us understand the distribution of contaminants.
Equilibrium Assumptions and Evaporation Considerations
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The analysis assumes no evaporation occurs, and equilibrium is achieved between the solid sludge and the water. Both the sludge and the slurry should retain the same concentration of A after the settling is completed.
Detailed Explanation
We are operating under the assumption that no material is lost to evaporation, which simplifies our calculations. By achieving equilibrium, we assume that mixing has allowed all materials to evenly distribute, and that once settled, the concentrations will not change. This means that when we take a sample for analysis, it should reflect the state of the system accurately.
Examples & Analogies
Think of a jar of salad dressing, where the oil and vinegar separate after standing still for a while. If you shake the jar (mixing), and then let it settle, the mixture will return to a similar state as before you mixed it. This is analogous to how the chemicals in the slurry will reach an equilibrium state.
Components of Mass Balance Equation
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The mass balance equation is structured as follows: Mass of A in sludge before equilibrium equals mass of A in water after equilibrium plus mass of A left in sludge.
Detailed Explanation
In the mass balance equation, we systematically account for where the mass of chemical A exists at different stages. Before equilibrium, all of A is in the sludge. After mixing and draining, we need to account for where that mass has gone. This transition allows us to derive equations to quantify A's distribution accurately.
Examples & Analogies
If you think of a party where balloons represent the mass of A; when you first fill a room with balloons (the sludge), and then remove some balloons to another room (the water), you realize the total number of balloons is conserved; they just moved from one room to another, similar to how the mass balance conserves the chemical A.
Complications from Chemical A’s Nature
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The rainwater introduced may not be pure; thus, additional considerations about contamination from the environment and the presence of organic carbon are important.
Detailed Explanation
It's noted that rainwater could potentially contain other chemicals (like pollutants) that can complicate our mass balance. The organic carbon's presence further complicates this, as it could absorb or interact with chemical A in ways not initially modeled. Therefore, we must be cautious about our assumptions.
Examples & Analogies
Consider when it rains in an urban area after a long dry spell; the first rain washes away dirt and pollutants from pavements into the gutters. Similarly, rainwater may introduce unexpected pollutants into our sludge mix, making it harder to predict how the chemicals are distributed.
Key Concepts
-
Mass Balance: A principle used to account for all mass in a system, ensuring conservation during processes.
-
Equilibrium Conditions: State of balance between phases in a system where no net change occurs.
-
Chemical Distribution: How substances separate and distribute themselves within different phases of a mixture.
Examples & Applications
In an industrial setting, a waste tank containing sludge undergoes mixing when rainwater is added, altering the concentrations of chemicals present.
The concept of mass balance is applied to determine how much of a chemical can be found in the aqueous phase versus the solid phase post-mixing.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In a tank with sludge so thick, mass balance is the trick!
Stories
Once upon a time, a tank full of sludge received a rainfall. The mixture swirled, and a wise engineer balanced the loads, ensuring nothing was lost but rather transferred between phases.
Memory Tools
M.E.P. (Mass, Equilibrium, Phase) – remember to track these three in mass balance.
Acronyms
B.A.S.E. (Balance, Assume, Solve, Equilibrate) - steps to follow for mass balance calculations.
Flash Cards
Glossary
- Mass Balance
A principle that states mass cannot be created or destroyed in a closed system; it must be conserved over time.
- Suspension
A mixture where solid particles are dispersed in a liquid but are not dissolved.
- Equilibrium
A state in which all forces or influences are balanced, often referring to the mixing and settling of substances.
- Moisture Content
The amount of water present in a substance, typically expressed as a percentage of the total mass.
- Chemical Loading
The amount of a specific chemical present per unit mass of the solid material.
Reference links
Supplementary resources to enhance your learning experience.
