Interphase Mass Transfer – Boundary Layer and Mass Transfer Coefficient
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Fundamentals of Mass Transfer
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Good morning class! Today, we will dive deeper into interphase mass transfer. Can anyone tell me what the driving force behind mass transfer is?

Is it the concentration difference?

Exactly! The driving force for mass transfer is often a difference in concentration, which induces flux. This driving force can also be related to chemical potential. Let’s remember this with the acronym 'CE', where ‘C’ stands for Concentration and ‘E’ for Equilibrium. When we discuss equilibrium, we mean how far a system is from that balance.

What happens if there is no velocity in the fluid?

Great question! If there’s no velocity, diffusion dominates mass transfer. So, in a stagnant fluid, mass transfer relies solely on concentration gradients.

How do we measure these fluxes?

We use the mass transfer coefficient ‘k’! It quantifies how effective the transfer is under the conditions we have. Remember, k is essential in stating how fast material moves across the boundaries.

Can k change based on the material?

Absolutely, Student_4! The value of k changes based on factors such as the interface type and flow conditions. Let’s summarize: The driving force is concentration difference, flux is driven by both convection and diffusion, and k is our key quantitative measure.
Velocity and Concentration Boundary Layers
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s move onto the concept of boundary layers. Who can explain what a boundary layer is?

Isn’t it the area near a surface where the flow velocity changes?

Yes! That's correct. The boundary layer affects both velocity and concentration gradients. Close to the surface, flow slows down due to friction, forming a velocity gradient that influences mass transfer.

What about concentration? Does it also form a boundary layer?

Absolutely! As fluid approaches the surface, a concentration gradient develops, leading to what we refer to as the concentration boundary layer. As the fluid moves, an increase in concentration happens as more mass transfers into the fluid.

So, the thinner the boundary layer, the better the mass transfer?

Yes, Student_3! A thinner boundary layer means less resistance and allows for more effective mass transfer. Remember! Think of the phrase 'Keep It Thin for Quick Flow' (KITTQF) to remind yourself of the importance of minimizing boundary layer thickness.
Role and Calculation of Mass Transfer Coefficient
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s focus on the mass transfer coefficient. Why is it crucial in our calculations?

It helps predict how much mass will transfer over time!

Exactly! It's vital for predicting mass transfer rates in various engineering processes. We typically define it in relation to the flux and driving force as: Flux = k * (Driving Force).

Does k have different forms for different systems?

Right again! K can vary depending on the system. For example, mass transfer coefficients can differ between air-water, air-solid, or soil-liquid interfaces. It’s crucial to be precise with our notation.

How do we actually calculate k in practice?

In practice, measuring k often involves experimental trial and error or detailed computational models that incorporate fluid dynamics and thermodynamics. Connecting these concepts is rather complex, yet it's important for accurate modeling.

So to summarize: k helps us measure mass transfer efficiency, and it changes across different systems?

Precisely! You've all grasped the core concepts involved. Remember, understanding k allows us to predict and optimize processes in environmental engineering.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, key concepts such as the driving force of mass transfer, the development of velocity and concentration boundary layers, and the significance of the mass transfer coefficient in predicting mass transfer rates are covered. The discussion emphasizes the balancing act between convection and diffusion in influencing mass transfer processes across different phases.
Detailed
Detailed Summary
In this section, we explore the essential principles of interphase mass transfer, specifically focusing on the boundary layer and mass transfer coefficient. The driving force for mass transfer is typically a difference in chemical potential, often related to concentration gradients that manifest during this process.
Key Points:
- Mass Transfer Mechanisms: Mass transfer occurs through two primary mechanisms—convection and diffusion. While diffusion always happens due to concentration differences, convection becomes more prominent in fluid systems with increased velocity, leading to chaotic motion and the formation of eddies.
- Boundary Layer Formation: At interfaces between two phases, a boundary layer forms where fluid dynamics influence mass transfer. This layer experiences velocity gradients from the effect of friction at surfaces, which can be laminar or turbulent depending on flow conditions.
- Concentration Gradient: As fluid flows through this region, a concentration gradient develops, creating an additional resistance to mass transfer characterized by a concentration boundary layer.
-
Mass Transfer Coefficient (k): The mass transfer coefficient is a critical parameter that quantifies the relationship between the driving force (concentration gradient) and mass flux. It varies depending on the interface and conditions, and various notations are used to represent different phases in contact.
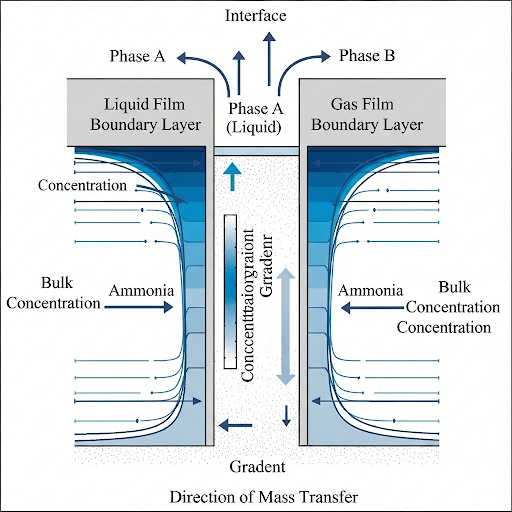
Understanding these concepts is crucial in environmental quality monitoring and analysis, facilitating better modeling of mass transfer processes in engineering applications.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Driving Forces in Mass Transfer
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, one of these 2 terms has to be an equilibrium related term, we will get to that, and the resistance is related to the actual transport of this molecule, how quickly or how well efficiently it can move from one place to another place.
Detailed Explanation
In mass transfer, the process depends on driving forces and resistances. A driving force often refers to a concentration difference, which indicates how far a system is from equilibrium. If one area has a higher concentration of a substance than another, this difference will drive the movement (or flux) of that substance. Resistance is about how quickly that substance can move from one place to another, influenced by factors like the fluid's properties and molecular interactions.
Examples & Analogies
Think of it like a crowded room where people are trying to move towards an exit. The driving force is the fact that there are more people near the exit than further away – they feel compelled to move that way. Resistance is like the obstacles (like furniture or other people) that slow their movement. Just as some might find an easier path to the exit, in mass transfer, some molecules find it easier to move than others.
Types of Mass Transfer: Diffusion and Convection
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When there is a little bit of motion, the term increases in magnitude; at some point this will become more than the diffusion because this will dominate.
Detailed Explanation
Mass transfer can occur via two main mechanisms: diffusion and convection. Diffusion happens when molecules move from an area of higher concentration to an area of lower concentration, and is driven by concentration gradients. On the other hand, convection is the movement of molecules due to bulk fluid motion, which can enhance mass transfer rates. As fluid velocity increases, convection effects can become more significant compared to diffusion, facilitating quicker transport of molecules.
Examples & Analogies
Imagine stirring sugar into your coffee. Initially, you can see sugar granules settled at the bottom – that's diffusion. However, once you stir, convection kicks in, rapidly mixing and distributing the sugar throughout the coffee. This rapid mixing through stirring mirrors how convection can enhance mass transfer in fluid systems.
Boundary Layer Concept
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The velocity profiles will start forming here, and there is a development of this velocity profile will be like this, initially it will look like this, very little friction is happening.
Detailed Explanation
The boundary layer is the region near a surface where the effects of friction and viscosity are significant in influencing fluid motion. When fluid flows past a surface, the velocity is highest away from the surface but slows down as it gets closer due to friction. This creates a gradient in velocity, where the closest layer to the surface may be nearly stationary, and the fluid is experiencing different speeds as you move away from the surface into the fluid. This has implications for mass transfer because the resistance to transfer is highest in this boundary layer.
Examples & Analogies
Consider a boat moving in a lake. The water very close to the bottom of the boat moves slower due to friction with the hull of the boat, while the water further away moves faster. The slower water forms a 'boundary layer' that influences how effectively the boat moves through the water, much like how the flow near a surface influences mass transfer.
Mass Transfer Resistance and the Concentration Gradient
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This mass transfer is a result of, the way mass transfer occurs in this region is a result of... the concentration gradient.
Detailed Explanation
Mass transfer resistance is influenced by the concentration gradient which exists across the boundary layer. As the fluid flows over a surface, a concentration gradient will develop, characterized by high concentration at the surface and lower concentration further away. This gradient drives the mass transfer, with the rate being dictated by how steep the gradient is and the extent of the resistance in the surrounding fluid. The steeper the gradient, the faster the mass transfer occurs.
Examples & Analogies
Think about a sponge being placed in water with a concentration of colored dye. Initially, the surface of the sponge absorbs dye quickly because there's a high concentration of dye in the water compared to the sponge. As the sponge is saturated, the concentration gradient lessens, slowing the rate of absorption, mirroring how concentration gradients affect mass transfer rates.
Convective Mass Transfer Coefficient
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In our scheme of nomenclature, simply the nomenclature is k of A in a phase... different kinds of mass transfer.
Detailed Explanation
The convective mass transfer coefficient (k) quantifies the mass transfer efficiency of a substance across an interface due to convection. This coefficient varies depending on the system and conditions, such as fluid velocity and the characteristics of the interfaces involved. It helps engineers predict how quickly a substance will transfer from one phase to another, guiding design and optimization in various processes.
Examples & Analogies
Imagine a busy airport with travelers using moving walkways to speed up their journey. The walking speed of travelers represents the convective mass transfer coefficient; the faster they walk (like a higher velocity flow), the more quickly they get to their destination (like faster mass transfer). This coefficient helps measure how effective the walkways are in transporting people, similar to how k helps in evaluating mass transfer rates.
Key Concepts
-
Driving Force: The concentration difference that drives mass transfer processes.
-
Boundary Layer: A region near a surface where fluid velocity and concentration gradients develop.
-
Mass Transfer Coefficient (k): A proportionality factor for estimating mass transfer rates.
Examples & Applications
Example 1: When a droplet of dye is placed in still water, the color spreads due to diffusion, showing how concentration gradients influence mass transfer.
Example 2: In a pipe, when water flows over a rough surface, turbulence enhances mass transfer rates compared to smooth surfaces.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Moving mass, oh what a scene, Diffusion flows and convects in between.
Stories
Imagine a river rushing past a plant. The water represents the fluid, and the roots symbolize molecules transferring nutrients.
Memory Tools
Remember 'FAD' - Flux, Area, and Diffusion, as essentials in understanding mass transfer.
Acronyms
Use ‘BASIC’ for Boundary layer, Advection, Surface interaction, Interface, and Concentration gradient.
Flash Cards
Glossary
- Mass Transfer Coefficient (k)
A parameter that quantifies the rate of mass transfer between phases, dependent on specific geometrical, physical, and operational conditions.
- Boundary Layer
The region near a surface where the velocity of the fluid changes due to friction.
- Flux
The rate of mass transfer per unit area, typically influenced by concentration gradients.
- Convection
The transport of mass by bulk fluid movement.
- Diffusion
The movement of mass from an area of higher concentration to an area of lower concentration.
- Concentration Gradient
The change in concentration of a substance between two regions.
Reference links
Supplementary resources to enhance your learning experience.
