Environmental Quality: Monitoring and Analysis
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Mass Transfer
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s start with the basics of mass transfer. Can anyone tell me what mass transfer involves?

Is it about how materials move from one place to another?

Exactly! Mass transfer is the movement of substances driven by concentration gradients. Why do you think knowing about mass transfer is important in environmental contexts?

It helps us understand how pollutants spread!

Good point! Monitoring how chemicals disperse helps us evaluate their environmental impact. Remember this: **Molecular diffusion** can happen even without mixing!

So, if something dissolves slowly in water, it’s still moving due to diffusion?

Exactly! That’s the essence of mass transfer. Let’s summarize: Mass transfer involves movement driven by gradients, essential for monitoring pollutants.
Dispersion Modeling
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s dive into dispersion modeling. What do you think it helps us to predict in environmental quality?

It might help predict how chemicals spread in the environment?

Correct! Dispersion modeling calculates how contaminants move through different mediums like air and water. Can someone give me an example of this?

An oil spill would be a good example!

Yes! When an oil spill occurs, lighter oil floats, while heavier chemicals may sink. It's crucial to estimate the **rate of release** to assess the spill's environmental impact.

How do we calculate that rate?

The rate of release is determined by factors like medium, chemical properties, and environmental conditions. Remember, understanding these rates is key for effective environmental monitoring. Let's summarize these points on dispersion modeling!
Environmental Interaction Scenarios
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s talk about specific scenarios where mass transfer plays a critical role. Can anyone suggest a situation?

What about a chemical leak into a river?

Good example! Chemicals can either dissolve in water or settle to the bottom. Over time, how might these chemicals spread?

They could spread downstream or mix with sediment!

Exactly! The chemical can slowly release into the water column as it diffuses back into the river. This highlights the importance of monitoring sediment over time. Let’s summarize the key scenarios we’ve discussed.
Understanding Chemical Potential in Mass Transfer
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To deepen our understanding, let’s examine **chemical potential**. How does this relate to mass transfer?

Is it about the energy that drives the movement of chemicals?

Exactly! Chemical potential is a thermodynamic driver behind diffusion and mass transfer. Why does it matter for our topic?

Understanding it helps us predict how and why substances move!

Correct! The movement driven by different potentials leads to active mass transfer until equilibrium is reached. Remember this: movement is essentially a drive for freedom among molecules. Let’s recap the significance of chemical potential.
The Importance of Monitoring Chemical Releases
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Why is it critical to monitor chemical releases into the environment?

To prevent major pollution or damage to ecosystems!

Exactly! Without monitoring, we may not recognize the lasting impact of substances that eventually spread, even if they're invisible initially. Can someone give an example of a remediation effort that could be done?

We might do cleanup operations for spills.

Absolutely! Monitoring helps determine if cleanup is necessary or if leaving it could be less harmful. Effective strategies stem from understanding mass transfer dynamics. Let’s summarize the significance of monitoring chemical releases!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section discusses the fundamentals of mass transfer and dispersion modeling in environmental contexts, specifically how chemicals disperse in different systems such as air, water, and soil. It provides examples of mass transfer scenarios, including oil spills and chemical leaks, and highlights the significance of understanding these processes for environmental impact assessment.
Detailed
Environmental Quality: Monitoring and Analysis
Overview of Concepts
This section explores the fundamentals of mass transfer related to environmental quality and monitoring, particularly through dispersion modeling and its applications in various systems such as air, water, and soil. The goal is to clarify how materials (like chemicals) move between different phases and interfaces in the environment.
Mass Transfer Fundamentals
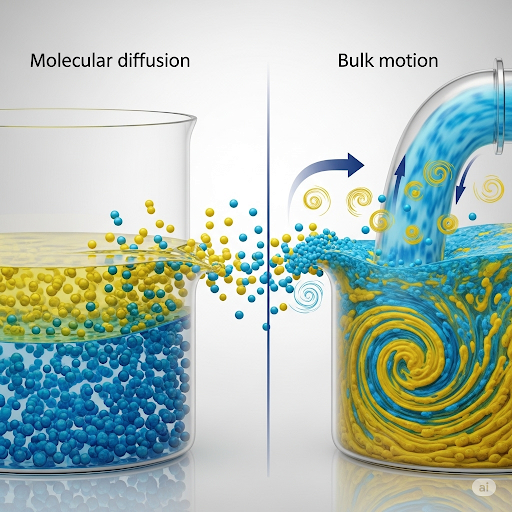
- Mass Transfer involves the movement of substances from one location to another, influenced by various forces like concentration gradients.
- The relation between diffusion and bulk motion is established, emphasizing that while mixing enhances speed, substances will still transfer due to molecular diffusion.
Dispersion Modeling
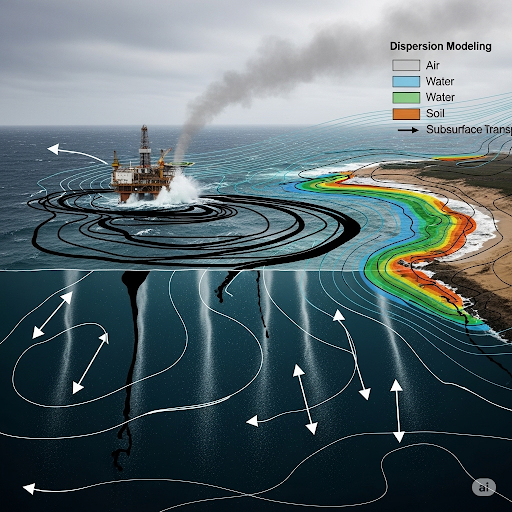
- Dispersion modeling is described as a technique to predict the distribution of substances in environmental systems. It incorporates factors such as the type of medium (air, water, soil) and properties of the substance involved (e.g., density, solubility).
- Key examples include the behavior of oil spills, where lighter oil floats on water while heavier chemicals sink and may spread in sediment.
- Understanding how these dynamics unfold over time is crucial for assessing ecological impacts.
Conclusion
This section stresses the importance of monitoring environmental quality through understanding mass transfer processes in various contexts, highlighting that even invisible chemicals can significantly impact ecosystems over time.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Mass Transfer Concepts
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In this section, we will discuss the fundamentals of mass transfer and its application in environmental quality monitoring. We'll begin by understanding the basic terms and concepts, focusing on mass transfer mechanisms such as dispersion modeling and interface mass transfer.
Detailed Explanation
Mass transfer is the movement of different substances into and out of phases, such as air, water, and soil. It is crucial in understanding environmental scenarios like pollution dispersal. In dispersion modeling, we calculate how substances spread in the environment, while interface mass transfer refers to the exchange of substances across different phases. Both concepts are foundational for assessing the impact of chemicals released into the environment.
Examples & Analogies
Consider a drop of food dye in a glass of water. Initially, the dye is localized where it was added, but over time, it spreads throughout the water. This process illustrates mass transfer—particularly diffusion—where the dye molecules move from a region of high concentration to low concentration until they are evenly distributed.
Understanding Q: Rate of Release of Chemicals
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
To understand environmental impacts, we measure Q, the rate of release of a chemical from a source. The source can be various surfaces such as water, soil, or air. This rate is influenced by factors like the chemical's properties and the medium it interacts with.
Detailed Explanation
Q represents how quickly a chemical leaves its source and enters the environment, which is vital for determining the potential risk associated with chemical spills. For example, in a water body affected by an oil spill, understanding how quickly components of the oil evaporate or dissolve helps predict how the spill will affect the ecosystem over time.
Examples & Analogies
Imagine a sponge soaking up water. The rate at which the sponge absorbs water can be likened to Q. If you place a dry sponge in a pool, it will quickly soak up water (high Q), but once it is saturated, the rate slows down and eventually stops, illustrating how a source can become overwhelmed.
Effects of Oil Spills on Water Bodies
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Specifically looking at oil spills, when oil, which is less dense than water, floats on the surface, some components will evaporate while others dissolve. Knowing the rates of these processes helps assess the potential environmental impact.
Detailed Explanation
Oil spills not only lead to visible pollution but also involve complex interactions with the environment, such as evaporation and dissolution. Understanding these interactions is essential to forecast the environmental damage and to decide on the most effective clean-up strategies. For example, measuring how quickly certain compounds evaporate from the oil helps in risk assessment and remediation planning.
Examples & Analogies
Think of a spilled cup of milk on a table. If left alone, some milk might evaporate, while some might seep into the cracks of the table. Recognizing how much milk can be cleaned up versus what will remain is akin to assessing how much oil from a spill can be removed through evaporation or dissolution.
Chemical Behavior in Sediments
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When chemicals sink into sediments, they can spread and dissolve. Over time, these chemicals may re-enter the water column, complicating their monitoring and study.
Detailed Explanation
Chemicals that settle into sediment can have a delayed impact on water quality, as they do not immediately cause harm. Over time, these chemicals can diffuse back into the water, which makes monitoring difficult since their presence might not be immediately visible. Understanding this behavior is essential for effective environmental assessments.
Examples & Analogies
Imagine dropping a colored crystal in a pond. Initially, it sinks and leaves the water clear. But after some time, it dissolves in the water, causing the pond to change color. This illustrates how contaminants can behave in sediments and later affect the water quality.
Transport Fundamentals Overview
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The final part discusses how mass transfer concepts apply to various environmental scenarios, including soil-air interactions and the movement of contaminants.
Detailed Explanation
Understanding transport fundamentals allows us to assess how pollutants travel through different mediums, including air and soil. The transport mechanisms such as diffusion, bulk movement, and the characteristics of the medium play significant roles in determining how and when pollutants will affect the environment.
Examples & Analogies
Consider the way scents spread in a room. When you light a scented candle, the aroma gradually fills the space. This is a diffusion process; similarly, pollutants diffuse through air or water, illustrating how substances can travel through different environments and potentially impact ecological systems.
Key Concepts
-
Dispersion Modeling: A technique to predict how pollutants spread in the environment across various mediums.
-
Chemical Potential: A concept explaining the driving force behind the movement of substances during diffusion.
-
Molecular Diffusion: The natural movement of molecules due to concentration differences, occurring even without mixing.
Examples & Applications
Example of an oil spill where lighter oil floats while heavier chemicals sink, demonstrating different environmental behaviors.
Chemical spill in a river illustrating how certain chemicals dissolve in water while others contaminate the sediment.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In rivers and lakes, chemicals break, spreading out, for the environment's sake!
Stories
Imagine a mischievous chemical spill that wanted to visit every corner of the river! It floated on top but also sank below, spreading everywhere and affecting all it encountered.
Memory Tools
Remember 'MATERIALS' for mass transfer: Movement, Air, Rivers, Temperature, Interfaces, Absorption, Leakage, Solubility.
Acronyms
DICE for dispersion
Diffusion
Interactions
Concentration
Environment.
Flash Cards
Glossary
- Mass Transfer
The movement of substances from one location to another driven by concentration gradients.
- Dispersion Modeling
A technique used to predict the distribution and movement of substances within various environmental media.
- Chemical Potential
A thermodynamic property that serves as a driving force for mass transfer.
- Molecular Diffusion
The passive movement of molecules from an area of higher concentration to an area of lower concentration without external forces.
- Bulk Motion
The movement of substances in a medium as a result of external forces, such as stirring or fluid flow.
- Concentration Gradient
A difference in the concentration of a substance across space, which drives diffusion.
Reference links
Supplementary resources to enhance your learning experience.
