Introduction to Interphase Mass Transfer
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Basics of Mass Transfer
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s start by defining mass transfer. Can anyone tell me what mass transfer is?

Isn't it the movement of mass from one place to another?

Exactly! Mass transfer refers to the movement of mass from one location to another, often between different phases. Can anybody give me an example of mass transfer?

An example could be sugar dissolving in water.

Good one! Remember, mass transfer can occur through different mechanisms, like diffusion or convection. We’ll deep dive into these mechanisms shortly.

What's the difference between diffusion and convection?

Great question! Diffusion is the movement of particles from an area of higher concentration to lower concentration without external forces, while convection involves the bulk movement of fluids.

To help remember these concepts, think of 'D' for Diffusion and 'C' for Convection – D moves slow (driven by concentration), while C moves fast (driven by flow).

So let’s summarize: Mass transfer is essential in environmental contexts, involving different phases and mechanisms. Remember the key terms: diffusion, convection, and their differences.
Interphase Mass Transfer Concepts
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand the basics, let’s look at interphase mass transfer. Can anyone explain what interphase mass transfer is?

Is it the transfer between different states, like gas to liquid?

Spot on! Interphase mass transfer involves the movement of mass between two different phases. A classic example is an oil spill where oil floats on water. What happens to the oil over time?

Some of it evaporates while some dissolves in the water.

Yes! This is a key aspect of monitoring environmental impacts. We calculate the rate of release, often termed as Q. Any thoughts on what influences this rate?

I assume the chemical properties like density and solubility would play a role?

Exactly! Density and solubility affect how substances migrate in different environments. Let’s not forget the importance of understanding the time factor in these calculations.

Summarizing, interphase mass transfer encompasses the movement between phases, greatly influencing environmental monitoring. Remember, density, solubility, and evaporation rates!
Diffusion and its Fundamentals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s delve into diffusion—a core concept in mass transfer. What do you think drives diffusion?

Is it concentration differences?

Right! Diffusion occurs because particles move from higher to lower concentration regions. Can anyone explain why this is essential in our mass transfer discussions?

Because it governs how substances like pollutants move?

Exactly! Understanding diffusion allows us to predict environmental impacts more accurately. Let’s remember this: 'Higher concentration drives higher diffusion' — a phrase to keep in mind.

As we wrap up, note that diffusion underpins many critical environmental processes, particularly with pollutants. Remember: Concentration Difference equals Movement.
Practical Applications in Environmental Monitoring
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s connect our concepts to real-life situations. How do you think these mass transfer principles apply to a river with a toxic chemical spill?

I think the chemical would diffuse in the river and could contaminate fish habitats.

Great observation! Mass transfer principles dictate how quickly and widely that contamination spreads. What might we need to monitor?

We should monitor concentrations over time.

Yes, tracking concentrations helps us understand pollutant behavior and implement effective mitigation strategies.

As we summarize this session, remember: Monitoring is vital. Use diffusion laws to predict movement in environmental scenarios.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section elaborates on interphase mass transfer principles, emphasizing examples such as oil spills and chemical dispersion in various environments. It explains how rate calculations, molecular diffusion, and bulk movement affect environmental monitoring and analysis.
Detailed
Introduction to Interphase Mass Transfer
This section explores the concept of interphase mass transfer, particularly its role in environmental quality monitoring and analysis. The discussion begins with an overview of mass transfer fundamentals, particularly in reference to dispersion modeling as a subset of interphase mass transfer.
Key points include:
- The basic concept of mass transfer and its significance in chemical release from different phases (e.g., air, water, soil).
- Specific examples, including oil spills, where the density of substances affects their behavior during spillage.
- Rate of chemical release, illustrated through various contexts such as water to air and air to soil transfers, showcasing the complexity of environmental impacts.
- The significance of understanding molecular diffusion as a fundamental principle driving mass transfer, as well as the differences between bulk movement and molecular diffusion.
- The notion of chemical potential and its relationship with concentration differences, which driving the tendency of chemicals to diffuse.
- An exploration of flux and diffusion coefficients, underscoring the factors influencing these measurements.
Overall, the section provides a comprehensive understanding of mass transfer processes and their implications for environmental engineering.
Youtube Videos


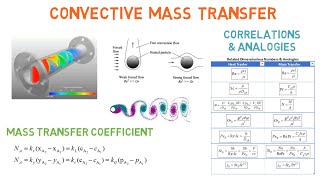




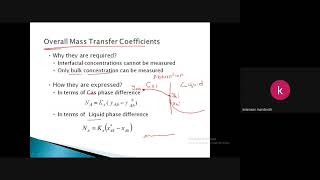

Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Mass Transfer Concepts
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, in the box model one of the things that we have we discussed is you know what will happen, the dispersion modeling was a slight departure from what we are doing in the box model. So, dispersion modeling is one subset of this, we are not exactly following the box model concept in its totality. We are using the box inside a plume and then we are integrating that, but here in box model we are using it as trying to calculate for the entire system here. So, one component of this is interface mass transfer, we will discuss that.
Detailed Explanation
In this introductory chunk, the speaker explains the concept of mass transfer within two frameworks: the box model and dispersion modeling. The box model represents a simplified system used for calculations, while dispersion modeling is introduced as a more complex representation that includes multiple variables like plumes. The speaker highlights that the focus will be on interphase mass transfer, which involves the exchange of material across different phases (e.g., from liquid to gas). Understanding these concepts is crucial for determining how pollutants or chemicals behave in different environmental scenarios.
Examples & Analogies
Think of the box model like a simple container that holds different ingredients for a recipe, while dispersion modeling is akin to cooking those ingredients together, allowing them to mix and interact more thoroughly. If you're baking cookies, the box model is just mixing flour and sugar in a bowl. Dispersion modeling is when you actually put the mixture in the oven, allowing it to change shape and texture, much like chemicals dispersing in an environment.
Understanding Rate of Release (Q)
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So let’s talk about mass transfer concepts fundamentals. We will talk a little bit about fundamentals, then we will look at the application where we are okay. So what we are really interested in finding out here is again this. So, if you take the example of the dispersion model, if you need to calculate the term Q, Q is the rate of release of a chemical A from a surface which has some chemical okay.
Detailed Explanation
This chunk delves into the concept of 'Q’, the rate at which a chemical (labeled A) is released from a particular surface. Understanding this rate is essential when modeling how chemicals interact with the environment, especially when they dissolve into water, evaporate into the air, or escape into soil. The rate of release is affected by various factors, including the chemical’s properties, environmental conditions, and the type of surface it is interacting with. Thus, calculating 'Q' helps predict the behavior and potential impacts of pollutants.
Examples & Analogies
Imagine a sponge pouring water onto a countertop. The flow of water (Q) from the sponge to the countertop symbolizes how chemicals are released from surfaces in the environment. If the sponge is full (a high concentration of chemical), the spill will be larger, much like a greater rate of release. If the countertop has a strong inclination (similar to a certain environmental condition), the water will flow more rapidly, representing how external factors influence chemical release.
Impact of Oil Spills
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For example simple examples of this say we have water, we have air, and here we have an oil spill let us say we have an oil spill, okay. If you assume that the oil has a density, which is less than that of water. The density of the oil is less than that of water, it will float on water okay.
Detailed Explanation
In this example, the speaker describes an oil spill scenario to showcase the principles of mass transfer in real-world situations. Since oil is less dense than water, it will float on the water's surface. Over time, certain components of the oil will evaporate into the air while others may dissolve into the water. This information is crucial for environmental scientists as they assess the potential impacts of the spill and how to mitigate its effects. Understanding the mass transfer processes involved can help predict the long-term consequences of such environmental accidents.
Examples & Analogies
Consider a salad dressing with oil and vinegar. When you pour it onto a salad, the oil floats on top of the vinegar. Similarly, in an oil spill, the oil will sit on top of the water, and over time, some may evaporate (like the vinegar's aroma) while some may mix with the vinegar. This illustrates how different components can interact with their environment in various ways.
Chemical Behavior in Water and Sediment
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Another example of this is, I have a boat that is carrying a lot of chemical, there is sediment which is under water, we had discussed this. The density of the chemical is greater than that of water, then it will not float, it will sink.
Detailed Explanation
This chunk discusses a different scenario where a chemical that is denser than water will sink to the bottom, impacting sediment and the surrounding environment. Unlike oil spills, which are visible on the surface, this chemical interaction occurs beneath the water surface where it can spread through sediment. Over time, the chemical can dissolve back into the water or travel downstream, making it difficult to track its movement and potential environmental impact.
Examples & Analogies
Imagine dropping a rock into a pond. Just like the rock sinks and settles to the bottom, certain chemicals will also sink into the sediment. Over time, just as the rock might slowly be worn away by water, the chemical may leach back into the water. This interaction highlights how unseen processes in the environment can greatly affect ecosystems.
Importance of Monitoring and Mass Transfer Fundamentals
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, I want to give you a brief overview on the transport fundamentals, it will probably take one or two lectures on this, where it is easier for you to appreciate how the calculations are done.
Detailed Explanation
In this closing chunk, the speaker emphasizes the necessity of understanding mass transfer fundamentals in environmental monitoring and analysis. These principles help in making informed decisions regarding the management of pollutants and chemicals. A thorough grasp of these concepts is essential for future lessons and for practical applications in environmental engineering and chemistry.
Examples & Analogies
Consider a baking class where understanding the properties of ingredients is crucial for successfully creating a cake. Just like bakers must know how to mix ingredients properly to ensure the cake rises, environmental scientists need to understand mass transfer to manage pollutants effectively.
Key Concepts
-
Interphase Mass Transfer: The movement of substances between phases.
-
Diffusion: The process of moving molecules from high to low concentration.
-
Chemical Potential: The energy involved in a chemical species that drives its movement.
-
Flux: A measurement of flow per area that relates to chemical movement.
Examples & Applications
An oil spill in a river where oil floats on water and gradually evaporates and dissolves.
Chemicals released from soil into the air during a contamination incident.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When particles flow, from high to low, that's diffusion, now you know!
Stories
Imagine pouring a drop of ink in water. At first, it stays concentrated, but soon, like magic, it spreads out evenly. That’s diffusion in action!
Memory Tools
D.C. for 'Diffusion Concentration'—remember, diffusion happens where there's differences in concentration!
Acronyms
D-M-C
Diffusion
Mass transfer
Chemical Potential—covering the essentials in one go!
Flash Cards
Glossary
- Interphase Mass Transfer
The movement of mass between different phases, such as solid, liquid, and gas.
- Diffusion
The net movement of particles from an area of higher concentration to an area of lower concentration.
- Concentration
The amount of a substance per defined space or volume.
- Chemical Potential
The potential energy of a chemical species that dictates its movement in a system.
- Flux
The rate of flow of a property per unit area.
Reference links
Supplementary resources to enhance your learning experience.
