SECONDARY POLLUTANTS
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Pollutants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to talk about pollutants in our atmosphere. Can anyone tell me what a primary pollutant is?

Is that the type that comes straight from a source, like car exhaust?

Exactly! Primary pollutants, like carbon monoxide and sulfur dioxides, are directly emitted from sources. And what about secondary pollutants?

Are those created from interactions in the atmosphere?

Correct! Secondary pollutants form when primary pollutants undergo chemical reactions in the presence of sunlight or other atmospheric components. A classic example is ozone. What do we know about ozone?

Ozone can heal high up in the atmosphere but is harmful when it's near the ground!

Yeah! It can cause respiratory issues, right?

Great points! Hence, while ozone protects us from UV rays high above, ground-level ozone is a concern. To remember this, think of 'Ozone's Dual Life!'
Effects of Secondary Pollutants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss the impacts of secondary pollutants. Why do we care about them?

Because they affect our health!

Absolutely! Secondary pollutants like smog can lead to respiratory problems, eye irritation, and more. Does anyone know the historical significance of smog in cities?

I read that London had major smog events in the past!

Right! The Great Smog of 1952 led to many deaths. This shows how crucial it is to monitor these pollutants. So, remember, 'Smog Stifles Cities.'

What about acid rain? Is that linked to these pollutants?

Yes, it is! When SOx and NOx mix with moisture, they lead to acid rain, which can devastate forest ecosystems. That's why we say 'Acid Rain, Ecosystems Drain.'
Prevention and Control of Secondary Pollutants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s wrap up our session today by discussing control measures for secondary pollutants. How can we help decrease their levels?

Reducing vehicle emissions could help, right?

Absolutely! Less emissions lead to less primary pollutants, which means less secondary pollutants too. What about industrial processes?

They should use cleaner technologies!

Exactly! Cleaner technologies minimize emissions. Can anyone give me a hint about reducing ozone levels specifically?

Fight back with plants that absorb those pollutants?

Right! Tree canopies can also help filter out some pollutants. So remember, 'Control Pollution, Protect Air Quality!'
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Secondary pollutants result from atmospheric reactions of primary pollutants and include harmful substances such as ozone, smog, and acid rain, which pose significant risks to human health and the environment. Understanding these pollutants is crucial for implementing effective air quality measures.
Detailed
Secondary Pollutants
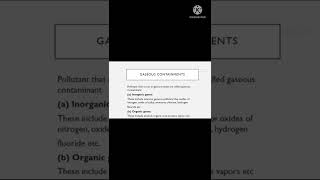
Secondary pollutants form through complex chemical reactions in the atmosphere, primarily involving primary pollutants emitted directly from sources such as vehicles and industrial areas. Notable examples of secondary pollutants include:
- Ozone (O3): Produced when sunlight reacts with pollutants such as volatile organic compounds (VOCs) and nitrogen oxides (NOx). While ozone in the upper atmosphere protects us from UV radiation, ground-level ozone can cause serious respiratory issues.
- Smog: A mixture of smoke and fog that can lead to severe air quality issues, particularly during warm months when temperature inversions trap pollutants near the ground.
- Acid Rain: Results when sulfur oxides (SOx) and nitrogen oxides (NOx) mix with atmospheric water vapor; it can severely harm ecosystems and buildings. Acid rain has pH levels below that of normal rain, leading to detrimental effects on soil, waterways, and aquatic life.
Overall, understanding secondary pollutants is crucial as they play a significant role in air quality impacts and subsequent control measures.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Ozone Depletion
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Ozone consists of oxygen molecules which contain three oxygen atoms. It is not emitted directly into the air but produced in the atmosphere when oxygen combines with oxygen radical (O�). Ozone protects us from ultra violet radiation and other harmful rays. It is observed that over the last few years, many manmade processes release gases into atmosphere causing drastic depletion of ozone layer. The chlorine atoms cause depletion of ozone slowly and holes are formed in the ozone layer. Ozone reacts with tissues and cause for breathing and decrease the working ability of the lungs, chest pains and coughing. It lowers the human body resistance power and leads to cold; pneumonia also. Antarctic Ozone depletion: According to NIMBUS-7 satellite picture which was taken on 5th October, 1987 the protective ozone layer showed a hole over 50% of the area of the Antarctica continent covering 7 million sq km. On Jan 1st 1989, the country Montreal (Canada) proposed redesigning refrigeration, air conditioning technology replacing the use of CFCs by ozone friendly substitutes.
Detailed Explanation
Ozone is a special form of oxygen that has three oxygen atoms instead of the usual two. It forms in the atmosphere when oxygen molecules interact with sunlight. This ozone layer is crucial for life on Earth because it absorbs harmful ultraviolet radiation from the sun. Unfortunately, human activities have led to the release of gases, such as CFCs, that damage the ozone layer, creating holes that can allow dangerous UV rays to reach the surface. Over time, exposure to these rays can lead to serious health issues, including respiratory problems and a weakened immune system. For example, the Antarctic ozone depletion was so severe that satellites showed a significant hole in the ozone layer in 1987, prompting international action to phase out harmful substances.
Examples & Analogies
Think of the ozone layer as a protective sunscreen for the Earth. Just as sunscreen shields our skin from harmful UV rays while allowing us to enjoy the sun safely, the ozone layer protects our planet from the sun's stronger rays. If this sunscreen wears off, like the ozone layer is depleting, we become vulnerable to sunburns and skin damage, which can lead to serious health issues.
Effects of Ozone Depletion
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Ozone reacts with tissues and cause for breathing and decrease the working ability of the lungs, chest pains and coughing. It lowers the human body resistance power and leads to cold; pneumonia also.
Detailed Explanation
When ozone levels increase due to pollutants and depletion occurs, it can cause adverse health effects. High concentrations of ozone irritate the lungs and can reduce lung function, making it hard to breathe, causing chest pain, and leading to coughing. Prolonged exposure reduces immunity, increasing the risk of respiratory infections such as colds and pneumonia. This explains why areas with high levels of ozone pollution often report more respiratory issues among their residents.
Examples & Analogies
Imagine trying to breathe while running in a smoky, polluted room. The irritants in the air would make it difficult for your lungs to function properly, similar to how ozone pollution affects lung health. Just like we try to avoid smokey environments to protect our breathing, we must be aware of the ozone levels in our environment to protect our health.
Smog Formation
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Smog is a combination of smoke and fog or various gases when react in the presence of sunlight. The effects of smog on human health cause for respiratory, irritation to the eyes, diseases related to nose, throat, bronchitis, pneumonia, headache, nerves, liver, and kidneys. The first smog related deaths were recorded in London in 1873, when it killed 500 people. In 1892, December, London had worst experiences causing 1000 deaths. In 1940’s severe smog began covering the cities of Los Angeles in USA.
Detailed Explanation
Smog forms when smoke from fires, industrial emissions, or car exhaust combines with fog and sunlight, leading to a thick haze. This haze can seriously harm health by irritating the respiratory system and causing various diseases, such as bronchitis and pneumonia. Historical examples, like the London smogs in the late 19th century, show how deadly smog can be, resulting in hundreds of deaths due to respiratory distress.
Examples & Analogies
Consider a time when you walked through a thick fog and found it hard to see. Now imagine that this fog is mixed with smoke from cars and factories. Just as that fog blocks your view, smog blocks clean air and can make breathing difficult, much like how wearing a mask would help in polluted environments.
Acid Rain
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Acid rain has become one of the most important global environmental problems and poses significant adverse impact on soils, rivers, lakes, forests and monuments. The phenomenon occurs when SOx and NOx from the burning of fossil fuels such as Petrol, Diesel, Coat etc combine with water vapour in atmosphere and fall as rain or snow or fog. Natural sources like volcanoes, forest fires, etc also contribute SOx and NOx. Increased urban and industrial activities cause air pollution resulting in the rise of concentration of SO and NOx. Sulphur dioxide and NO combine with water vapour in the atmosphere produce sulphuric acid and Nitric acid respectively and results acid rain. Some of the examples are: Europe and parts of W Asia have experienced rain with water pH range of 4.5 to 5.0 (acidic) in 1958.
Detailed Explanation
Acid rain is a significant environmental issue caused by sulfur oxides (SOx) and nitrogen oxides (NOx) released from burning fossil fuels. These gases combine with moisture in the atmosphere to form acids, which can then fall as rain, snow, or fog with a lower pH, meaning it's more acidic. This type of precipitation can harm various ecosystems, soil health, and even structures like monuments by corroding them. Regions like Europe experienced dangerously acidic rain, indicating how widespread and impactful acid rain can be.
Examples & Analogies
Think of acid rain like a strong lemonade spill on a picnic table. Just like the acidity in lemonade can cause wood to warp or corrode over time, acid rain can damage forests, rivers, and even buildings, showcasing the importance of maintaining a clean environment to protect our shared spaces.
Key Concepts
-
Primary Pollutants: Emitted directly from sources and can include gases like CO and SOx.
-
Secondary Pollutants: Formed by chemical reactions in the atmosphere, including ozone, smog, and acid rain.
-
Ozone Layer: Beneficial in the upper atmosphere but harmful at ground level due to health risks.
Examples & Applications
Ground-level ozone contributes to smog and respiratory problems for people in urban environments.
Acid rain has been shown to contribute to the degradation of forest ecosystems and historical monuments.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Ozone's got two faces, heals us above; but down with pollution, it's not what we love.
Stories
Once in a distant city, smog descended like a heavy blanket, causing coughing fits among the residents. They learned that less pollution meant clearer skies and healthier lungs.
Memory Tools
Remember 'O.S.A': Ozone, Smog, Acid rain - these are secondary pollutants.
Acronyms
Think of 'P.A.S.S' - Primary for emissions, Acid rain for nature, Smog for cities, Sec for secondary.
Flash Cards
Glossary
- Secondary Pollutants
Pollutants that are not emitted directly but form in the atmosphere through reactions between primary pollutants and other atmospheric substances.
- Ozone
A gas composed of three oxygen atoms; it can be beneficial in the stratosphere but harmful as a secondary pollutant at ground level.
- Smog
A type of air pollution that combines smoke and fog, leading to poor air quality and health issues.
- Acid Rain
Rain that is more acidic than normal due to pollutants such as sulfur oxides and nitrogen oxides mixing with water vapor.
Reference links
Supplementary resources to enhance your learning experience.
