Boiling Heat Transfer
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Pool Boiling
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to explore pool boiling. Can anyone tell me what happens during pool boiling?

Is it when liquid water is heated until it bubbles?

Exactly! Pool boiling is the boiling of a stationary liquid on a heated surface. It involves several regimes such as natural convection boiling and nucleate boiling. Who can explain what nucleate boiling means?

I think it has to do with bubbles forming and breaking off the surface?

Correct! This bubble activity helps enhance heat transfer. Can anyone remember another boiling regime?

I remember transition boiling, where it becomes unstable?

Great recall! Transition boiling is indeed unstable, and then there’s film boiling, which occurs when a vapor film forms and reduces heat transfer. Sounds complex, right?

It does, but it helps to think about the different heat transfer rates!

Exactly. Remember, the boiling curve helps us visualize this. We'll wrap up by remembering that the critical heat flux is where heat transfer declines sharply!
Exploring Flow Boiling
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s move on to flow boiling. Can anyone summarize what flow boiling involves?

Is it when water flows over a heated surface?

Exactly! Flow boiling occurs when a liquid flows over a heated surface, like in many industrial systems. How does this differ from pool boiling?

Flow boiling combines forced convection with bubble formation, right?

Yes! It’s more complex because you have heat transfer from both the flow and the bubbles. What are some places where you think flow boiling is applied?

Maybe in boilers and power plants?

Spot on! Flow boiling is fundamental in those processes. Remember the role of turbulence in enhancing heat transfer!
Practical Applications of Boiling
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s think about real-world applications of boiling heat transfer. Can anyone share where this might be important?

In cooling systems for electronics?

Absolutely! Boiling processes are vital in electronics cooling. What about in energy systems?

Boilers and evaporators!

Correct! Boiling is also essential in heat pipes. It's exciting to see how these principles apply to so many industries! Reviewing your notes on the boiling curve will be helpful before our next class!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section discusses the fundamentals of boiling heat transfer, which includes pool boiling and flow boiling. It elaborates on the mechanisms involved, key heat transfer regimes, and their practical applications, with an emphasis on the boiling curve and critical heat flux.
Detailed
Boiling Heat Transfer
Boiling heat transfer is a critical process involving the phase change from liquid to vapor at a solid-liquid interface, leading to enhanced heat transfer rates due to the release of latent heat. The section is divided into key areas:
Pool Boiling
This refers to the boiling of a stationary liquid on a heated surface and includes various heat transfer regimes:
1. Natural Convection Boiling: Occurs at low temperature differences (∆T).
2. Nucleate Boiling: Characterized by bubble formation and detachment.
3. Transition Boiling: An unstable regime with partial film formation.
4. Film Boiling: Where vapor insulates the surface, resulting in lower heat transfer rates.
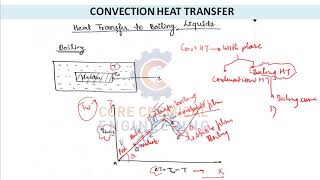
The boiling curve illustrates the relationship between heat flux and surface superheat (Ts−Tsat), indicating a critical heat flux (CHF) point beyond which heat transfer efficiency declines sharply.
Flow Boiling
This occurs when liquid flows over a heated surface, such as in tubes, and combines forced convection with nucleate boiling, making it more complex yet essential in systems like boilers and evaporators.
Applications
Boiling heat transfer is widely utilized in boilers, evaporators, cooling electronics, and heat pipes, making it vital for energy efficiency and thermal management.
Youtube Videos



Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Boiling Heat Transfer
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Boiling refers to phase change heat transfer from liquid to vapor, typically at a solid-liquid interface. It involves latent heat and is characterized by high heat transfer rates.
Detailed Explanation
Boiling heat transfer occurs when a liquid changes into vapor. This often happens at the interface where the liquid is in contact with a solid surface that is heated. The process uses latent heat, which is the energy needed for the phase change between liquid and vapor without altering the temperature. Because boiling can transfer a large amount of heat quickly, it is highly effective in various industrial applications.
Examples & Analogies
Think of heating water in a kettle. As the pot gets hot, the water at the bottom starts to boil, creating steam. This transformation from liquid to vapor is quick and efficient, which is why boiling is popular for cooking and generating steam in electricity plants.
Types of Boiling: Pool Boiling
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Pool Boiling
● Boiling of a stationary liquid on a heated surface
● Heat transfer regimes:
○ Natural convection boiling (low ∆T)
○ Nucleate boiling (bubbles form and detach)
○ Transition boiling (unstable, partial film formation)
○ Film boiling (vapor film insulates surface; lower heat transfer)
Detailed Explanation
Pool boiling occurs when a liquid is stationary and heated from below. There are different heat transfer regimes:
- Natural convection boiling happens at low temperature differences and relies on the natural movement of the liquid as it heats.
- Nucleate boiling is when bubbles form on the surface, detach, and rise through the liquid, enhancing heat transfer.
- Transition boiling shows instability where some regions may start to form a vapor film, reducing heat transfer efficiency.
- Film boiling occurs when a thick vapor film covers the surface, significantly isolating it and causing heat transfer rates to drop.
Examples & Analogies
Imagine boiling pasta in a pot. At first, the water heats up slowly (natural convection), then you see bubbles forming (nucleate boiling). If the heat increases too much, you might see a film of steam covering the bottom of the pot (film boiling), making it harder for the heat to escape into the water.
The Boiling Curve
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Boiling Curve:
● Heat flux vs. surface superheat (Ts−Tsat)
● Includes critical heat flux (CHF) point — beyond this, heat transfer drops drastically.
Detailed Explanation
The boiling curve plots the relationship between heat flux (the amount of heat transferred per unit area) and the superheat of the surface (the temperature difference between the surface and the saturation temperature of the liquid). The critical heat flux (CHF) is an important point on this curve, where beyond this temperature, the efficiency of heat transfer decreases sharply, potentially leading to system failure.
Examples & Analogies
Consider a radiator in your home. It works best when it operates within a certain temperature range. If the radiator gets too hot, it can start to fail and won't effectively provide heat anymore. This is similar to the boiling curve's critical point where too much heat leads to inefficiency.
Types of Boiling: Flow Boiling
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Flow Boiling
● Occurs when liquid flows over a heated surface (e.g., inside tubes)
● More complex due to combination of forced convection and nucleate boiling
● Common in boilers, evaporators, and power plants
Detailed Explanation
Flow boiling occurs when liquid is continuously moving over a heated surface, and this scenario is more complex than pool boiling. Here, forced convection from the flow of the liquid enhances the heat transfer in addition to nucleate boiling occurring at the solid-liquid interface. This is often utilized in industrial applications like boilers, evaporators, and power plants where liquids need to be heated efficiently while moving.
Examples & Analogies
Think about water flowing through a hot pipe. As the water moves, it doesn't just sit still to boil; it actively carries away the heat and forms bubbles along the way. This is similar to how currents in a river can help speed up the melting of ice rather than just waiting for the sun to warm the whole river.
Key Concepts
-
Boiling Heat Transfer: The process of transferring heat through phase change from liquid to vapor.
-
Pool Boiling: A type of boiling when the liquid remains stationary.
-
Flow Boiling: Involves heat transfer when liquid moves over a heated surface.
-
Critical Heat Flux: The threshold at which heat transfer efficiency drops significantly.
Examples & Applications
Boiling water in a kettle demonstrates pool boiling and nucleate boiling.
Flow boiling in industrial heat exchangers, like coolers in power plants.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In a kettle, or a pot, / Pool or flow, they boil a lot. / With bubbles, heat they share, / Critical flux shows we must beware.
Stories
Imagine you’re a drop of water in a kettle. As you heat up, you change from liquid to steam, racing to escape! This journey reflects pool boiling and how heat transfers to the air, especially before you reach critical flux.
Memory Tools
Remember 'NPTF': Nucleate, Pool, Transition, Film - It's the sequence of boiling regimes in pool boiling.
Acronyms
Use 'BC' for Boiling Curve - it helps you remember how heat flux changes with temperature.
Flash Cards
Glossary
- Boiling Heat Transfer
Phase change heat transfer from liquid to vapor, characterized by high heat transfer rates.
- Pool Boiling
Boiling of a stationary liquid on a heated surface, including various heat transfer regimes.
- Flow Boiling
Boiling that occurs when liquid flows over a heated surface, combining forced convection and nucleate boiling.
- Natural Convection Boiling
A heat transfer regime occurring at low temperature differences where buoyancy drives the flow of liquid.
- Nucleate Boiling
A boiling regime characterized by the formation and detachment of bubbles from a heated surface.
- Film Boiling
A boiling regime where a vapor film insulates the heated surface, resulting in lower heat transfer.
- Critical Heat Flux (CHF)
The maximum heat flux at which a phase change can occur without a significant drop in efficiency of heat transfer.
Reference links
Supplementary resources to enhance your learning experience.
