The Nature of Matter and Atomic Theory
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding the Atom
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to learn about atoms! Can anyone tell me what an atom is?

Isn't an atom like the tiny building block of everything?

Exactly! An atom is the smallest unit of an element that retains its properties. Now, does anyone know what atoms are made of?

They are made up of protons, neutrons, and electrons!

That's right! Protons are positively charged, neutrons are neutral, and electrons are negatively charged. A good way to remember this is via the acronym 'PNE'—protons, neutrons, and electrons.

What about the nucleus?

Great question! The nucleus is the dense center of the atom, containing protons and neutrons. It holds most of the atom's mass!

So, electrons are around the nucleus, right?

Yes! Electrons orbit the nucleus in energy levels, like planets around the sun. Now, can anyone remind me why the atomic number is important?

Because it tells us how many protons there are?

Exactly! The atomic number is key to identifying the element. Great work, everyone! To summarize, an atom consists of protons, neutrons, and electrons, with the nucleus at its center.
Historical Models of the Atom
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

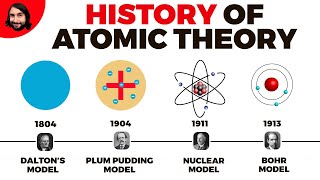
Let's discuss the history of atomic theory. Who can tell me about Democritus?

He was a Greek philosopher who thought matter was made of tiny particles he called 'atomos.'

Correct! He proposed that these particles were indivisible. Later, in 1808, John Dalton built on this idea with his atomic theory. What did Dalton believe?

He said atoms are indivisible and combine in whole number ratios?

Exactly! Now, onto discoveries in the 19th and 20th centuries. Can anyone tell me who discovered the electron?

It was J.J. Thomson in 1897!

Right! Thomson used cathode rays and proposed the 'plum pudding model.' Following that, who discovered the proton?

Ernest Rutherford in 1917?

Correct! And who can tell us about the neutron?

James Chadwick discovered it in 1932 to explain the mass of the nucleus!

Excellent summary, everyone! Remember, understanding these discoveries helps us appreciate modern atomic theory.
Atomic Number and Isotopes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, who can remind me what the atomic number signifies?

It's the number of protons in the nucleus!

Exactly! Let’s explore how this relates to isotopes. Can anyone give me an example of isotopes?

Carbon-12 and Carbon-14!

Yes! Both are forms of carbon but differ in neutrons. Carbon-12 has 6 protons and 6 neutrons, while Carbon-14 has 6 protons and 8 neutrons.

So they have the same atomic number but different mass numbers?

Exactly! The atomic number tells us the element, while the mass number indicates the total count of protons and neutrons.

Why do isotopes matter?

Isotopes have significant applications in fields like medicine and carbon dating. Remember, isotopes share chemical properties but can differ in stability and mass.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
What is an Atom?
Chapter 1 of 1
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
An atom is the smallest unit of an element that still retains the properties of that element. It is made up of three types of subatomic particles:
• Protons (p+): Positively charged particles found in the nucleus.
• Neutrons (n0): Neutral particles with no charge, also located in the nucleus.
• Electrons (e−): Negatively charged particles that orbit the nucleus in various energy levels.
Detailed Explanation
An atom is essentially the fundamental building block of matter. It retains the unique characteristics of the element it represents. Within the atom, there are three main types of particles:
1. Protons: They have a positive charge and are found in the center of the atom, known as the nucleus. The number of protons determines the identity of the element (for example, hydrogen has one proton while oxygen has eight).
2. Neutrons: These particles have no charge and also reside in the nucleus. Neutrons contribute to the mass of the atom but do not affect its charge. Different variants of an element, known as isotopes, can have varying numbers of neutrons.
3. Electrons: These are the negatively charged particles that move around the nucleus in specific allowed zones called energy levels. The behavior and arrangement of electrons are critical as they play a significant role in chemical reactions and bonding.
Examples & Analogies
Think of an atom like a miniature solar system. The nucleus, containing protons and neutrons, is like the sun at the center, where most of the mass is concentrated. The electrons are like planets that orbit around the sun, but they don’t follow specific paths like planets do; instead, they occupy areas where they are likely to be found.
Key Concepts
-
Atom: The smallest unit of an element, retaining its chemical properties.
-
Subatomic Particles: Protons, neutrons, and electrons make up atoms.
-
Atomic Number: Determines the identity of an element based on the number of protons.
-
Mass Number: The total number of protons and neutrons in the nucleus.
-
Isotopes: Variants of an element having the same number of protons but different neutrons.
Examples & Applications
Hydrogen is an atom with 1 proton and 1 electron; its atomic number is 1.
Carbon-12 has 6 protons and 6 neutrons, while Carbon-14 has 6 protons and 8 neutrons, illustrating isotopes.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Protons are positive, neutrons are neutral, electrons zing around, like a whirlpool.
Stories
Once in a tiny world, there lived particles: proud protons, neutral neutrons, and energetic electrons, each finding their place in the dance of atoms.
Memory Tools
PNE for Protons, Neutrons, Electrons is a quick way to remember the subatomic components.
Acronyms
Remember 'AIN' for Atomic number (A), Isotope (I), and Neutron (N) to connect atomic concepts.
Flash Cards
Glossary
- Atom
The smallest unit of an element that retains its chemical properties.
- Proton
A positively charged particle found in the nucleus of an atom.
- Neutron
A neutral particle found in the nucleus of an atom.
- Electron
A negatively charged particle that orbits the nucleus.
- Atomic Number
The number of protons in an atom's nucleus, defining the element.
- Mass Number
The sum of protons and neutrons in an atom's nucleus.
- Isotope
Atoms of the same element with different numbers of neutrons.
Reference links
Supplementary resources to enhance your learning experience.
