Le Châtelier’s Principle
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Le Châtelier's Principle
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to talk about Le Châtelier's Principle, which helps us understand how systems at equilibrium react to external changes. Can anyone tell me what they think equilibrium means?

I think equilibrium means that the forward and reverse reactions happen at the same rate.

Exactly! In a closed system, the concentrations of reactants and products remain constant over time. Now, Le Châtelier’s Principle states that if we apply a stress to this system, like changing concentrations, the system will adjust to counteract that stress. Does anyone have an example of this?

If we add more reactants, the reaction should shift to produce more products, right?

Spot on! This counteraction helps to reach a new equilibrium. Let's remember this with the acronym 'REACT' – 'React to External Adjustments of Concentration and Temperature.'
Effect of Concentration Changes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's dive deeper into how changes in concentration affect equilibrium. What happens if we add a product or remove a reactant?

If we add a product, the equilibrium will shift to the left, towards the reactants.

Correct! This shift helps to minimize the increase in product concentration. And if we were to remove a reactant?

Then the equilibrium would shift to the left towards the reactants to replace what was lost.

Fantastic! Let's summarize this with the mnemonic 'RAISE and REDUCE' – Add raises, remove reduces products or reactants!
Effect of Pressure Changes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let's discuss how pressure changes affect gas phase equilibria. Who can explain what happens when we increase pressure?

Increasing pressure shifts the equilibrium to the side with fewer gas molecules.

Right! It’s to reduce the pressure effect!

Exactly! Now what about decreasing pressure?

That should shift the equilibrium towards more gas molecules.

Great job! Remember this with the phrase 'Less Pressure, More Volume.'
Effect of Temperature Changes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s explore how temperature affects equilibria. Who can provide the rule for what happens when we increase temperature?

Increasing temperature shifts the equilibrium toward the endothermic side of the reaction.

Correct! And what about lowering the temperature?

That would favor the exothermic direction.

Exactly! Let’s remember this with 'HOT goes to COLD' - heat shifts to the cold side. This helps you recall which direction the equilibrium will shift!
The Role of Catalysts
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let’s discuss the role of catalysts. How do they influence an equilibrium system?

They speed up the rate at which equilibrium is reached but don’t change the equilibrium position.

So, they lower the activation energy for both sides equally?

Exactly! Remember with the phrase 'Catalysts Keep it Steady and Swift!', indicating they do not alter the position of equilibrium.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Le Châtelier’s Principle states that if an external change is applied to a system at equilibrium, the system will adjust itself to counteract that change. This section explores the effects of changes in concentration, pressure, and temperature on equilibrium, and how catalysts influence the equilibrium process.
Youtube Videos



Audio Book
Dive deep into the subject with an immersive audiobook experience.
Statement of Le Châtelier’s Principle
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Le Châtelier’s Principle: If an external stress (change in concentration, pressure, or temperature) is applied to a system at equilibrium, the system adjusts (shifts) in such a way as to partially counteract that stress and reestablish equilibrium. In other words, the equilibrium “moves” to oppose the change imposed.
Detailed Explanation
Le Châtelier’s Principle explains how a system at equilibrium responds when it experiences disturbances or stresses, such as changes in concentration, pressure, or temperature. When these changes occur, the equilibrium of the system shifts in a direction that partially counteracts the disturbance, striving to return to a balanced state. For example, if more reactant is added, the equilibrium will shift towards producing more products to consume the extra reactant.
Examples & Analogies
Think of a crowded room where one person suddenly opens a door letting in fresh air. The people (representing the reactants) might settle more towards one side, creating a new balance while still feeling the breeze. In the same way, the chemical reactions adjust to respond to the 'extra air' (change), trying to maintain their balance.
Effect of Concentration Changes
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Adding or removing a reactant or product:
- If you add more reactant, the reaction will shift to the right (toward products) to consume some of the added reactant and restore equilibrium.
- If you remove reactant, the equilibrium shifts to the left (toward reactants) to replace some of the removed species.
- Similarly, adding product shifts the equilibrium to the left; removing product shifts equilibrium to the right.
Example: For the general reaction a A + b B ⇌ c C + d D, if we suddenly double [A], the reaction will proceed in the forward direction (to the right) until a new set of concentrations is reached at which Kc = ([C]^c [D]^d)/([A]^a [B]^b) again. The “extra” A gets used to form more C and D.
Detailed Explanation
This chunk discusses how changes in the concentration of reactants or products affect the equilibrium state of a reaction. When we add more of a reactant, the system tries to balance itself by consuming that extra reactant to produce more products. Conversely, if some reactant is removed, the system will shift back to produce more reactant to compensate for the loss. The same logic applies to products, where adding products shifts the equilibrium towards reactants, and removing products shifts it towards products.
Examples & Analogies
Imagine you're at a party, and everyone is dancing (reactants and products). If suddenly more friends arrive (adding reactants), the group might spread out and engage more with the new arrivals (creating products). If some friends leave (removing reactants), the remaining dancers might stop dancing and wait for more friends to return before they start dancing again.
Effect of Pressure Changes (for Gas-Phase Equilibria)
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
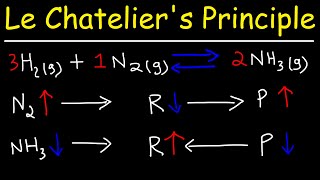
Increasing total pressure by volume reduction: When gases are compressed (volume decreases), partial pressures of each gaseous species increase. According to Le Châtelier’s Principle, if the net mole count of gaseous species on the product side differs from that on the reactant side, the equilibrium shifts to the side with fewer moles of gas to reduce total pressure.
Example (Haber process): N₂(g) + 3 H₂(g) ⇌ 2 NH₃(g)
- Initially, consider 1 mol N₂ and 3 mol H₂ (4 mol gas total) producing 2 mol NH₃ (2 mol gas total).
- If you suddenly increase pressure (compress the mixture), the system shifts to the side with fewer moles (2 mol of NH₃) to reduce pressure. Hence, higher pressure favors ammonia formation.
Detailed Explanation
Changes in pressure particularly affect gas-phase equilibria according to Le Châtelier's Principle. When the total pressure in a system is increased, the system will respond by shifting the equilibrium toward the side of the reaction that has fewer moles of gas, which reduces the overall pressure. This is particularly relevant in industrial applications like the Haber process for ammonia production, where increasing pressure leads to more ammonia being produced, as the reaction produces fewer moles of gas than it consumes.
Examples & Analogies
This can be likened to a balloon. If you squeeze a balloon (increase pressure), the air inside has to respond somehow. It can either compress to take up less space or force some of the air out through tiny openings (shifting reaction). In a chemical context, the 'squeezing' encourages the formation of products from reactants that take up less room.
Effect of Temperature Changes
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Temperature changes affect equilibrium in two distinct ways:
1. They change the value of K (the equilibrium constant) itself, because K is temperature‐dependent.
2. They cause the system initially at equilibrium to shift either forward or backward until it reaches a new equilibrium consistent with the new K.
- Endothermic versus exothermic reactions:
-
For a general reaction, represent the heat term on the appropriate side:
Exothermic reaction: heat is released in the forward direction. We write it as: A + B ⇌ C + D + heat.
or, equivalently, A + B → C + D ΔH is negative (heat released).
Endothermic reaction: heat is absorbed in the forward direction. We write: heat + A + B ⇌ C + D or A + B → C + D ΔH is positive (heat absorbed). - Raising temperature: Adding heat to the system: If the forward reaction is endothermic (requires heat), adding heat shifts equilibrium to the right (to consume the added heat). K (for that reaction) increases, favoring product formation. If the forward reaction is exothermic (releases heat), adding heat shifts equilibrium to the left (to consume some of the added heat). K decreases, favoring reactants.
- Lowering temperature: Removing heat has the opposite effect: equilibrium shifts toward the exothermic side.
Detailed Explanation
Temperature changes can significantly impact the equilibrium state of a reaction. When the temperature is raised, it can change the equilibrium constant K. If the reaction is endothermic, increasing the temperature favors the formation of products (right shift), while for exothermic reactions, it favors the reactants (left shift). Conversely, lowering the temperature has the opposite effect. When we think about it in terms of heat, we see that reactions either 'consume' or 'release' heat, which helps predict how temperature changes affect them.
Examples & Analogies
Consider a recipe for baking cookies. If you increase the oven temperature (raising it), the cookies (products) fry quicker because they need heat. If you lower the temperature, the cookies might collapse and not cook properly (favoring the reaction that leads to ingredients rather than finished cookies). The key is finding that perfect temperature where the cookies are just right.
Effect of Catalysts
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Catalysts increase the rate at which equilibrium is achieved by lowering the activation energy for both the forward and reverse reactions equally.
Important point: While catalysts speed up how fast equilibrium is reached, they do not change the position of the equilibrium or the value of K. The concentrations at equilibrium remain exactly the same as they would be without the catalyst; only the time to reach equilibrium is shortened.
Detailed Explanation
Catalysts are substances that speed up chemical reactions by lowering the energy barrier (activation energy) needed for the reaction to occur. This effect is true for both the forward and reverse reactions, allowing the system to reach equilibrium faster. However, it’s crucial to note that catalysts do not change the actual position of equilibrium or the equilibrium constant K; they simply make it possible to reach that state quicker. The concentrations of reactants and products at equilibrium remain the same, regardless of the presence of a catalyst.
Examples & Analogies
Think of catalysts like a friendly guide in a large museum. The guide helps you navigate the exhibits (the reaction process) faster. You still see the same paintings (equilibrium state), but with the guide, you spend less time wandering and arrive at your destination quicker.
Key Concepts
-
Le Châtelier’s Principle: A system at equilibrium will respond to changes by shifting to counteract the change.
-
Dynamic Equilibrium: The state where reactants and products interconvert at equal rates.
-
Equilibrium Constant (K): Indicates the ratio of products to reactants at equilibrium.
-
Impact of Temperature: Raising temperature can shift equilibrium depending on whether the reaction is exothermic or endothermic.
-
Effect of Catalysts: Catalysts accelerate the rate of reaction without shifting the equilibrium position.
Examples & Applications
If a reaction involves A + B ⇌ C + D, adding A will shift equilibrium to the right (toward products).
Increasing the pressure on 2CO(g) + O2(g) ⇌ 2CO2(g) will favor the right side because it has fewer gas molecules.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
If you raise the heat in a reaction that's real, products go back—it’s a common deal.
Stories
Imagine a factory trying to make cookies (products). If they get too many ingredients (reactants), they will adjust the recipe to balance once again.
Memory Tools
Think of ‘PETS’ - Pressure, Energy, Temperature, and Species, to remember what affects equilibrium!
Acronyms
Remember 'SHIFT' where 'S' if for Stress, 'H' for Heat changes, 'I' for Inert gases, 'F' for Favors, and 'T' for Temperature adjustments.
Flash Cards
Glossary
- Le Châtelier’s Principle
Principle stating that a system at equilibrium will shift to counteract any applied stress.
- Dynamic Equilibrium
State where the rates of the forward and reverse reactions are equal.
- Equilibrium Constant (K)
A numerical value that represents the ratio of concentrations of products to reactants at equilibrium.
- Exothermic Reaction
A reaction that releases heat, typically characterized by a negative ΔH.
- Endothermic Reaction
A reaction that absorbs heat, typically characterized by a positive ΔH.
- Activation Energy
The minimum energy required for a reaction to occur.
Reference links
Supplementary resources to enhance your learning experience.
