Applications of Redox Reactions
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Electrolysis and Electroplating
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we'll explore electrolysis and electroplating. Can anyone tell me what electrolysis is?

Isn't it when you use electricity to drive a chemical reaction?

Exactly! During electrolysis, an external current forces a nonspontaneous redox reaction to occur. Can anyone give an example of where we see this in action?

Electroplating is one, like when silver is deposited onto a copper object.

Great example! Would anyone like to elaborate on how this happens?

The copper is the cathode, and silver ions in solution are reduced to form silver metal on its surface.

Exactly! Remember, during electroplating, the metal ions are reduced at the cathode, creating a coating. Keep that in mind when thinking of applications!

How about why we need to use electrolytes in these processes?

Good question! Electrolytes help conduct electricity by providing ions. Without them, our electrolysis would not function effectively!

In summary, electrolysis uses electrical energy to cause a nonspontaneous redox reaction, leading to processes like electroplating and extraction of metals.
Batteries and Fuel Cells
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s move on to batteries and fuel cells. What do you think the main function of a battery is?

To store energy? When you need it, it converts that to electricity?

Correct! Batteries consist of galvanic cells that convert stored chemical energy into electrical energy. Can you tell me the difference between primary and secondary batteries?

Primary batteries can’t be recharged, right? Like alkaline batteries?

And secondary batteries can be recharged, like lithium-ion batteries!

Exactly! Now what about fuel cells? How do they differ from batteries?

Fuel cells continually consume reactants, while batteries store them.

Yes! And fuel cells offer high efficiency and only produce water as a byproduct when using hydrogen fuel. Remember the overall reaction in a hydrogen fuel cell!

To summarize, batteries store chemical energy and provide it as electrical energy, while fuel cells convert reactants into energy continuously.
Corrosion and Protection Methods
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Who knows what corrosion is? How does it relate to redox reactions?

It's the oxidation of metals, like when iron rusts.

Exactly! When iron oxidizes, it forms iron(III) oxide or rust. Can someone explain why protecting against corrosion is crucial?

It prevents structures from weakening and saves money on repairs!

Well said! So, what methods do we have for protecting metals?

We can use protective coatings like paint!

Or galvanization, where you coat a metal with a more easily oxidized one, like zinc.

Good job! Remember, zinc serves as a sacrificial anode to prevent rusting. Let’s recap: Corrosion is the oxidation of metals, and various methods can protect against it.
Applications in Biology
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s talk about biological processes. Can anyone name a metabolic process that relies on redox reactions?

Cellular respiration!

Yes! In cellular respiration, glucose is oxidized to produce carbon dioxide. What about the role of oxygen in the process?

Oxygen is reduced to form water!

Right! The controlled flow of electrons during these reactions allows cells to generate ATP, vital for life. What other process involves redox reactions?

Photosynthesis!

Absolutely! Water is oxidized, and carbon dioxide is reduced to create glucose. Let's summarize this: Both respiration and photosynthesis rely heavily on redox reactions, essential for energy conversion in biological systems.
Industrial Applications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s finish our discussion with industrial processes. Who can give me an example of an industrial application of redox reactions?

The Hall-Héroult process for extracting aluminum!

Yes! During this process, aluminum cations are reduced to aluminum metal at the cathode. What about in refining copper?

In electrolytic refining, impure copper is oxidized at the anode!

Exactly! Impurities are left behind, allowing you to acquire pure metal. Are there any other processes we should mention?

We should talk about the chlor-alkali process where brine is electrolyzed to produce chlorine gas!

Very well! Recapping, industrial applications of redox include aluminum extraction, copper refining, and chlorine production, showcasing the importance of these reactions in various fields.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Redox reactions have significant applications in fields like electrolysis, batteries, corrosion protection, analytical techniques, and biological processes such as respiration and photosynthesis. Understanding these applications provides insights into their impact on technology, environment, and life sciences.
Detailed
Detailed Summary
Redox reactions, which involve the transfer of electrons, are pivotal in various scientific and industrial processes. This section encapsulates their broad applications, highlighting their significance in multiple domains:
- Electrolysis and Electroplating: These processes utilize external electrical sources to drive nonspontaneous redox reactions. Electrolysis is vital for electroplating and extracting metals such as aluminum. The electroplating process involves depositing a metal layer onto a substrate by reducing metal ions from a solution.
- Batteries and Fuel Cells: Batteries consist of galvanic cells that convert chemical energy into electrical energy. Fuel cells provide a continuous energy supply by consuming fuel and oxidant, exemplifying efficient energy conversion without harmful emissions.
- Corrosion and Corrosion Protection: This section discusses how metals oxidize and form compounds like rust. Protecting metal surfaces using coatings, galvanization, and cathodic protection plays an essential role in minimizing corrosion.
- Analytical Techniques: Redox titrations are critical for quantifying chemical concentrations. Techniques such as permanganate titrations utilize color changes to indicate the equivalence point of a reaction. Other methods involve using indicators to detect redox changes in solutions.
- Biological Redox Processes: It highlights key metabolic processes like cellular respiration, where glucose is oxidized, and photosynthesis, where carbon dioxide is reduced. Both processes are fundamental to energy production in living organisms, emphasizing the associated redox reactions.
- Industrial Processes: Redox chemistry is crucial in metal extraction and purification, involving processes like the Hall-Héroult for aluminum production and electrolytic refining of copper, making steps efficient and sustainable.
Understanding these applications allows for insights into how redox chemistry impacts industry, the environment, and human health.
Youtube Videos
Audio Book
Dive deep into the subject with an immersive audiobook experience.
Electrolysis and Electroplating
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Electrolysis is a process in which an external electrical source forces a nonspontaneous redox reaction to occur. It is the reverse of a galvanic cell. Applications include:
- Electroplating: Deposition of a thin metal layer onto a substrate (another metal or nonmetal). For example, silver plating (silvering) a copper surface: a copper object is made the cathode in a silver salt solution (e.g., silver nitrate). When current passes, Ag+ ions are reduced to silver metal on the cathode surface, forming a shiny coating.
- Electrorefining: Purification of metals by making the impure metal the anode in an electrolytic cell. For instance, impure copper is oxidized to Cu2+ at the anode, then pure copper is deposited at the cathode, leaving impurities behind.
- Extraction of metals such as aluminum from alumina (Al2O3) dissolved in molten cryolite: aluminum cations are reduced to aluminum metal at the cathode, while oxide anions are oxidized to oxygen at the carbon anode. This is the industrial Hall-Héroult process.
In all electrolysis applications, one must choose appropriate electrolytes, electrode materials, and cell voltages to drive the desired redox reactions while minimizing side reactions (for instance, hydrogen or oxygen evolution).
Detailed Explanation
Electrolysis uses electricity to induce a redox reaction that normally would not happen spontaneously. Electroplating consists of placing an object in a solution containing metal ions. By applying current, metal from solution is deposited onto the item. Electrorefining cleans metals by oxidizing impurities while pure metal forms on another electrode. The Hall-Héroult process extracts metals like aluminum from ore by using electrolysis, which is crucial for making metals pure in a cost-effective manner.
Examples & Analogies
Think of electroplating like painting your nails: the nail surface (copper object) is coated with polish (silver coating) by adding color to it (metals). Just like different layers give your nails a shiny look, electroplating gives a metallic object a smooth, protective layer.
Batteries and Fuel Cells
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Batteries are compact galvanic cells (or assemblies of cells) that convert stored chemical energy into electrical energy on demand. Common types include:
- Primary (nonrechargeable) batteries: e.g., alkaline cells (zinc–manganese dioxide), alkaline-carbon cells, lithium primary cells. They undergo irreversible chemical changes and cannot be recharged.
- Secondary (rechargeable) batteries: e.g., lead-acid batteries (Pb–PbO2), nickel–cadmium (Ni–Cd), nickel–metal hydride (Ni–MH), lithium-ion (Li-ion) batteries. Applying an external current reverses the redox reactions, regenerating the reactants.
Example: Lead-acid battery half-reactions (in 1.2 M sulfuric acid):
- Discharge (galvanic) mode:
- Anode (oxidation): Pb(s) + SO4^2−(aq) → PbSO4(s) + 2 e−
- Cathode (reduction): PbO2(s) + 4 H+(aq) + SO4^2−(aq) + 2 e− → PbSO4(s) + 2 H2O(l)
Detailed Explanation
Batteries are devices that store and convert chemical energy into electricity. Primary batteries cannot be recharged, while secondary batteries can be recharged by reversing the chemical reactions that produce electricity. For example, in a lead-acid battery, reactions occur at the anode and cathode to either discharge or recharge the battery. This ability to store energy and release it on demand makes batteries essential for portable power.
Examples & Analogies
Think of batteries as gas tanks for energy. Just as a gas tank stores fuel for a car to drive, a battery stores energy for devices to operate. When you recharge a battery, it's like refilling your gas tank for another trip!
Corrosion and Corrosion Protection
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Corrosion is the undesired oxidation of metals, often resulting in metal oxide or hydroxide formation. Common examples include rusting of iron and tarnishing of silver. Understanding corrosion as a redox process allows strategies for protection:
- Protective Coatings: Paint, varnish, plastic coatings, or other impermeable layers prevent contact with oxygen and moisture.
- Galvanization: Coating iron or steel with a more easily oxidized metal (often zinc). Zinc acts as a sacrificial anode: it corrodes preferentially (zinc → Zn2+ + 2 e−), protecting the underlying iron.
- Cathodic Protection: Attaching a more negative (more easily oxidized) metal block (sacrificial anode) to a steel structure in contact with soil or water. The sacrificial anode dissolves instead of the protected metal.
- Alloying: Stainless steel (iron alloyed with chromium and nickel) forms a thin, passive chromium oxide layer that prevents further oxidation of iron.
- Design Considerations: Avoiding crevices where moisture can accumulate, preventing contact between dissimilar metals (which can cause galvanic corrosion), and controlling pH and electrolyte composition.
Understanding the half-reactions in corrosion (for example, iron oxidation to Fe2+, oxygen reduction to hydroxide) helps in designing inhibitors and protective measures.
Detailed Explanation
Corrosion is a natural process where metals like iron react with moisture and oxygen, leading to rust and decay. It is crucial to understand this process to protect metals, as it can lead to structural failures. Various strategies such as coatings, galvanization with zinc, or using sacrificial anodes help protect metals from corrosion. Prevention techniques alter how metal interacts with its environment, ensuring longevity and safety.
Examples & Analogies
Imagine a metal fence left out in the rain. Without protection, it rusts away as it oxidizes. Putting a coat of paint on it is like putting on sunscreen - it shields the metal from the harsh elements just as sunscreen protects skin from damaging sun.
Analytical Techniques: Redox Titrations and Indicators
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Redox titrations are volumetric analyses in which a solution of an oxidizing agent is titrated against a solution of a reducing agent (or vice versa) until the equivalence point, where stoichiometrically equivalent amounts have reacted. Common titrations include:
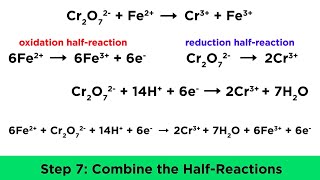
- Potassium permanganate titration: KMnO4 (a strong oxidizer) titrated against a solution containing Fe2+ (ferrous sulfate) or oxalate ions (C2O42–). Permanganate’s purple color disappears as it is reduced to colorless Mn2+, so the end point is the first persistent pale pink color. Reaction in acidic medium with Fe2+: 5 Fe2+ + MnO4− + 8 H+ → 5 Fe3+ + Mn2+ + 4 H2O
- Dichromate titration: K2Cr2O7 (potassium dichromate) is another common oxidizing titrant. For example, it can titrate Fe2+ in acidic solution by converting orange dichromate (Cr2O72–) to green Cr3+. An external indicator such as diphenylamine sulfonate may be used, since Cr3+ color change is not always sharp.
- Iodometry: Iodine/iodide redox titrations often involve generation of I2 by oxidation of I– with some oxidizer (e.g., Cu2+, ClO–), then titration of the liberated I2 with standardized thiosulfate solution. The end point is detected with starch indicator (blue-black complex with I2 disappears). Key steps for performing a redox titration include:
- Write the balanced redox reaction under the titration conditions (acidic, basic, neutral).
- Determine stoichiometry (moles of electrons exchanged per mole of analyte or titrant).
- Use a suitable indicator or a potentiometric method (measuring potential changes) to identify the end point.
- Calculate the analyte concentration from volume and concentration of titrant used at equivalence.
Detailed Explanation
Redox titrations are techniques to determine the concentration of a substance using redox reactions. One solution, which is either an oxidant or a reductant, is gradually added to another until they react completely. By monitoring the color change, you can ascertain when the reaction is complete and determine the concentration of the unknown solution based on stoichiometry.
Examples & Analogies
Think of redox titrations like filling a glass of water from a pitcher. As you pour water (the titrant) in, you notice the glass fill up (the reaction happening) until full (the equivalence point). Just as you can measure how much water you poured, you can measure how much titrant you need to find out how concentrated the solutions are.
Biological Redox Processes (Respiration, Photosynthesis)
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Living cells depend on controlled redox reactions to convert nutrients into usable energy (ATP). Two principal redox-driven processes are:
- Cellular respiration: Glucose is oxidized stepwise to carbon dioxide, and oxygen is reduced to water. Electrons travel through a chain of redox centers (NADH, flavins, iron-sulfur proteins, cytochromes, copper centers) in the mitochondrial inner membrane. This electron flow pumps protons across the membrane, creating a proton gradient. The return flow of protons through ATP synthase drives the synthesis of ATP from ADP and inorganic phosphate. Overall simplified equation: C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + energy (ATP + heat)
- Photosynthesis: In chloroplasts of plants and photosynthetic bacteria, water is oxidized (releasing O2) while carbon dioxide is reduced to carbohydrate. Light energy excites electrons in chlorophyll, which move through a series of redox carriers, pumping protons and generating a proton gradient. That gradient drives ATP synthesis. Electrons ultimately reduce NADP+ to NADPH, which is used in the Calvin cycle to fix CO2 into sugars. Overall simplified equation: 6 CO2 + 6 H2O + light energy → C6H12O6 + 6 O2
In both processes, the controlled movement of electrons through protein complexes and cofactors exemplifies redox chemistry in living systems.
Detailed Explanation
Biological systems rely heavily on redox reactions for energy transformation. In cellular respiration, glucose is broken down via oxidation, releasing energy stored in its bonds which is harnessed to produce ATP, our energy currency. Concurrently, during photosynthesis, plants harness light to oxidize water and reduce carbon dioxide, ultimately producing glucose. This interplay of oxidation and reduction is vital for life.
Examples & Analogies
Consider respiration in cells like charging your phone. Just as you plug your phone into a power source to replenish its battery, cells 'charge up' by breaking down glucose to produce ATP. In photosynthesis, think of plants as solar panels. Just as solar panels convert sunlight into electricity, plants convert sunlight into chemical energy, fostering life.
Industrial Processes (Extraction and Purification of Metals)
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Many large-scale industrial operations rely on redox chemistry:
- Hall-Héroult process: Extraction of aluminum metal from alumina (Al2O3) dissolved in molten cryolite. In a large electrolytic cell, aluminum cations gain electrons (are reduced) at the cathode to form molten aluminum metal, while oxide anions are oxidized at carbon anodes to form carbon dioxide.
- Electrolytic refining of copper: Impure copper is cast into an anode. In an electrolytic cell, Cu(s) from the anode is oxidized to Cu2+ and migrates to the cathode to deposit as pure copper. Impurities that are less noble (e.g., iron, zinc) remain in solution; those that are more noble (e.g., silver, gold) collect beneath the anode as anode sludge, which can be further processed.
- Electrolytic production of chlorine: In the chlor-alkali process, brine (sodium chloride solution) is electrolyzed. Chloride ions are oxidized at the anode to chlorine gas; water is reduced at the cathode to hydrogen gas and hydroxide ions. Resulting products are chlorine, hydrogen, and sodium hydroxide (caustic soda).
- Production of nitric acid: Ammonia is oxidized (using air and a platinum catalyst) to nitrogen monoxide, which is further oxidized to nitrogen dioxide, then absorbed in water to yield nitric acid. These are multiple redox steps essential to fertilizer production.
Industrial redox processes are engineered to maximize yield, minimize energy consumption, manage by-products, and control operational costs.
Detailed Explanation
Redox reactions play a crucial role in industry, particularly in the extraction and refinement of metals. The Hall-Héroult process is pivotal in obtaining aluminum, where electrolysis separates aluminum from its oxide. Similarly, electrolytic refining purifies copper by cycling it through oxidation and reduction reactions. Other industrial processes, such as the chlor-alkali and nitric acid production, rely on redox chemistry to convert raw materials into valuable products efficiently.
Examples & Analogies
Think of these industrial processes like making a favorite dish from raw ingredients. Just as cooking involves certain steps to transform raw vegetables and proteins into a delicious meal, industrial redox reactions meticulously transform raw materials (like ores) into usable metals and chemicals through a series of chemical changes.
Key Concepts
-
Electrolysis: A method of driving a chemical reaction using electrical energy to force nonspontaneous reactions, like in electroplating.
-
Batteries: Devices converting chemical energy into electrical energy, encompassing primary and secondary types.
-
Corrosion: The oxidation process that leads to metal degradation, requiring protective measures to mitigate its effects.
-
Fuel Cells: A clean energy technology generating electricity through redox reactions between fuel and oxidant.
-
Redox Titration: Analytical technique to determine the concentration of a solution through redox reactions.
Examples & Applications
Using electrolysis to plate a copper object with silver.
Using a lead-acid battery to power a car.
Rust formation on iron exposed to moisture.
Utilizing hydrogen fuel cells to power vehicles.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In electrolysis, we plug in the wire, driving reactions that ignite the fire!
Stories
Imagine a magician (Electrolysis) magically turning metal ions into shiny coins through the power of electric electricity.
Memory Tools
REDOX: Reduction is where electrons are gained, Oxidation is where they are lost.
Acronyms
B.E.C.F
Batteries store energy
Electroplating coats
Corrosion protects
Fuel cells generate.
Flash Cards
Glossary
- Electrolysis
A process that uses electrical energy to drive a nonspontaneous chemical reaction.
- Electroplating
The process of depositing a layer of metal onto a surface to enhance appearance or prevent corrosion.
- Corrosion
The chemical deterioration of a material due to reaction with its environment, often involving oxidation.
- Battery
A device that stores chemical energy and converts it into electrical energy.
- Fuel Cell
A device that generates electrical energy through the continuous consumption of fuel and oxidant via redox reactions.
- Redox Titration
A volumetric analysis technique that determines the concentration of an analyte using a redox reaction.
Reference links
Supplementary resources to enhance your learning experience.
