Oxidation and Reduction Reactions
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Defining Oxidation and Reduction
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're diving into oxidation and reduction reactions. Let's start with definitions. What does oxidation mean?

Isn't it about losing electrons?

Exactly, oxidation involves the loss of electrons, and when that happens, the oxidation number increases. Remember this mnemonic: 'LEO', which stands for 'Lose Electrons = Oxidation'. Now, what about reduction?

So, reduction must be gaining electrons, right?

Correct! We can remember it with 'GER': 'Gain Electrons = Reduction'. This is foundational for understanding redox reactions.

Can you give an example?

Sure! In a reaction between zinc and copper sulfate, zinc is oxidized as it loses electrons, while copper(II) ions are reduced as they gain electrons. Thus, we can see exactly how electron transfer occurs in these reactions.

Got it! So oxidation and reduction are always paired, right?

Exactly! They always occur together in what's known as redox reactions.

Key takeaways: Oxidation is losing electrons and increasing oxidation number, whereas reduction is gaining electrons and decreasing oxidation number.
Understanding Oxidation Numbers
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let's talk about oxidation numbers. What are they?

They help track how many electrons are gained or lost, right?

Exactly! They serve as a bookkeeping system. The rules for assigning oxidation numbers include that free elements have an oxidation number of zero. Can someone give me an example?

Like in O2, the oxidation number would be zero?

Spot on! Now, what about a monoatomic ion like Na+?

That would have an oxidation number of +1 since it's positively charged.

Perfect! Always remember these rules, especially the exceptions we discussed, such as oxygen typically being -2 but -1 in peroxides. What about the sum of oxidation numbers?

In neutral compounds, they add up to zero, right?

Exactly! And in polyatomic ions, they equal the ion's charge. This is essential for determining which substances are oxidized or reduced in reactions.
Identifying and Balancing Redox Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's identify oxidation and reduction in reactions. What steps do we take?

First, we assign oxidation numbers to all elements in the reactants and products.

Exactly right! And what do we look for next?

We compare the oxidation numbers from reactants to products to see who increases or decreases.

Correct! An increase means oxidation, while a decrease means reduction. Next, how do we balance these reactions?

We separate them into half-reactions, balance them for mass and charge, then combine them.

Well done! For example, in the reaction of iron and copper(II) sulfate, we see iron oxidized and copper reduced. Can anyone explain the steps clearly?

First balance the iron and copper separately, then make sure that the electrons lost equal those gained before combining.

Exactly! You must ensure mass and charge balance throughout. Remember, practice makes perfect!
Types of Redox Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s explore some common types of redox reactions! Who can tell me what a combustion reaction is?

It's when a fuel reacts with oxygen to produce heat and light, typically yielding carbon dioxide and water.

Exactly! Combustion can be complete or incomplete. What happens in incomplete combustion?

It can produce carbon monoxide instead of carbon dioxide!

Great job! Now, what about corrosion? How is it related to redox processes?

Corrosion involves metals reacting with oxygen and moisture, leading to metal oxides.

Exactly! Such as iron rusting. Lastly, what about biological redox reactions?

That’s where metabolism occurs, like cellular respiration!

Right again! Always think of redox in context to energy transfer in living organisms.
Balancing Redox Equations
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we have discussed different redox reactions, let’s focus on balancing the equations. What’s our first step?

We separate it into half-reactions, right?

Correct! Then what comes next?

We balance the atoms except for hydrogen and oxygen.

Exactly, and then we address oxygen and hydrogen by adding H2O and H+, respectively, in acidic solutions. What if we're balancing in basic media?

We must first balance as if it were acidic, then add OH- to neutralize H+.

Perfect! This methodical approach will help ensure that mass and charge balance in all cases.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, we delve into oxidation and reduction processes, where electrons are transferred between chemical species. Key concepts include definitions, oxidation numbers, how to identify which species is oxidized or reduced, and methods for balancing redox equations in both acidic and basic conditions.
Detailed
Oxidation and Reduction Reactions
Oxidation and reduction reactions, collectively known as redox reactions, involve the transfer of electrons between chemical species. This section defines oxidation as the loss of electrons (resulting in a higher oxidation number) and reduction as the gain of electrons (resulting in a lower oxidation number). Key highlights include:
- Definitions
- Oxidation refers to the loss of electrons by an atom, ion, or molecule, associated with an increase in oxidation number.
- Reduction is the gain of electrons, linked to a decrease in oxidation number.
- Oxidation Numbers: These serve as a bookkeeping method to track how many electrons are gained or lost during reactions. There are defined rules for assigning oxidation numbers, including that the oxidation state of free elements is zero, and for monoatomic ions, it's equal to their charge.
- Identifying Oxidation and Reduction: By assigning oxidation numbers, one can determine which species is oxidized (increased oxidation state) and which is reduced (decreased oxidation state). This allows for the separation of the reactions into half-reactions, which can then be balanced.
- Balancing Redox Equations: This covers techniques for balancing redox reactions in both acidic and basic solutions, ensuring that both mass and charge are conserved.
- Common Types of Redox Reactions: The section introduces combustion, corrosion, and biological metabolism as examples of redox processes that occur in various contexts. The knowledge gained is applicable across multiple scientific fields, making it essential for understanding both fundamental and complex chemical interactions.
Youtube Videos


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definitions of Oxidation and Reduction
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
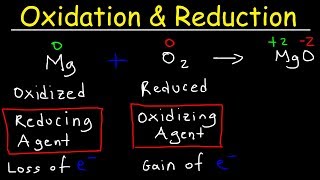
● Oxidation is the chemical process in which an atom, ion or molecule loses electrons.
○ When oxidation occurs, the oxidation number of that chemical species increases (becomes more positive or less negative).
○ The species that loses electrons is called the reducing agent because it donates electrons to another species.
● Reduction is the chemical process in which an atom, ion or molecule gains electrons.
○ When reduction occurs, the oxidation number of that chemical species decreases (becomes more negative or less positive).
○ The species that gains electrons is called the oxidizing agent because it accepts electrons from another species.
A simple mnemonic often used is “LEO the lion says GER”:
● Lose Electrons → Oxidation
● Gain Electrons → Reduction.
Detailed Explanation
This chunk introduces the fundamental concepts of oxidation and reduction, which are critical in redox reactions. Oxidation is defined as the loss of electrons, which results in an increase in the oxidation number of the species that undergoes this process. In contrast, reduction involves the gain of electrons, leading to a decrease in the oxidation number of the affected species. The reducing agent is the substance that donates electrons (gets oxidized), while the oxidizing agent accepts electrons (gets reduced).
To better understand this, think of a game of tag: the runner losing electrons is like someone who's tagged out (oxidation), while the tagger gaining those electrons (winner) becomes the new runner (reduction).
Examples & Analogies
Imagine a game where players can 'give' or 'take' balls to each other. If a player gives away a ball, they lose that ball and cannot play for a while (oxidation). Meanwhile, the player who just received the ball now has an advantage (reduction). The player who gives the ball is the reducing agent (since they’re reducing their number of balls), whereas the one receiving it is the oxidizing agent (as they’re gaining balls). This context helps visualize the movement of electrons during these chemical reactions.
Half-Reactions in Redox Reactions
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Every redox reaction can be thought of as two half‐reactions: one half shows oxidation (electron loss) and the other shows reduction (electron gain). For example, when metallic zinc is placed into a solution of copper(II) sulfate, zinc dissolves as zinc ions and copper metal is deposited:
1. Zinc metal → Zinc ion + Electrons
2. Copper(II) ion + Electrons → Copper metal
Writing these in full:
● Oxidation half‐reaction: Zn(s) → Zn²+(aq) + 2 e−
● Reduction half‐reaction: Cu²+(aq) + 2 e− → Cu(s)
When combined, the electrons cancel and yield the overall reaction:
Zn(s) + Cu²+(aq) → Zn²+(aq) + Cu(s).
In the overall reaction, zinc is oxidized (loses electrons) and copper(II) ions are reduced (gain electrons).
Detailed Explanation
In a redox reaction, you can visually split it into two distinct processes called half-reactions. The oxidation half-reaction involves the loss of electrons by a substance, while the reduction half-reaction involves the gain of those electrons by another substance. The example given involving zinc and copper(II) sulfate illustrates this well: zinc metal gets oxidized by losing electrons, and copper ions are reduced by gaining those electrons. When both half-reactions are combined, the electrons are canceled out, resulting in the overall reaction that shows the interchange of electrons.
This approach helps chemists analyze and balance reactions systematically. By understanding individual half-reactions, one can more easily identify changes in oxidation states and ensure mass and charge balance.
Examples & Analogies
Think about a relay race where one runner (the oxidizing agent) passes a baton (the electron) to another runner (the reducing agent). The runner who loses the baton must run back to get it, just like how the oxidizing agent loses electrons. The runner who receives the baton can now speed ahead, gaining an advantage, similar to how the reducing agent gains electrons. This analogy highlights how each runner's actions in the race reflect the oxidation and reduction processes in chemical reactions.
Oxidation Numbers and Assignment Rules
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Oxidation numbers (also called oxidation states) are a bookkeeping tool to keep track of electrons in redox reactions. An atom’s oxidation number is an assigned integer that represents its hypothetical charge if all bonds to atoms of different elements were ionic. The actual charge in covalent compounds differs, but the oxidation number helps identify which atoms are oxidized or reduced.
Rules for assigning oxidation numbers:
1. Free elements (uncombined atoms) have oxidation number zero.
2. Monoatomic ions have oxidation number equal to their charge.
3. Oxygen in most compounds has oxidation number −2.
4. Hydrogen usually has oxidation number +1 when bonded to non-metals, and −1 when bonded to metals.
5. Fluorine always has oxidation number −1 in compounds.
6. The sum of the oxidation numbers of all atoms in a neutral compound is zero.
Detailed Explanation
This chunk provides a way to assign oxidation numbers, which help keep track of the electrons involved in redox reactions. The oxidation number can often reveal the type of reaction occurring in a compound. For instance, free elements like oxygen and nitrogen have an oxidation state of zero, while monoatomic ions reflect their charge (like Na⁺ being +1). Specific rules help clarify the role of elements in compounds, particularly in understanding how oxidation and reduction occur. For example, in water (H₂O), oxygen's oxidation number is −2, leading to hydrogen's +1 to balance the charge.
These oxidation numbers serve as an essential tool for identifying which species have been oxidized (their oxidation number increases) and which have been reduced (their oxidation number decreases) in a reaction.
Examples & Analogies
Think of oxidation numbers as a game score that tracks players' (atoms') performance during a match. Different players (elements) may start with zero points (oxidation number zero) when the game begins, then as they make plays (react chemically), their scores change (oxidation number increases or decreases depending on whether they gain or lose electrons). Just like tracking a score ensures fair play and helps understand how the game is progressing, oxidation numbers help chemists understand how electrons are being exchanged during chemical reactions.
Identifying Oxidation and Reduction in Reactions
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
To determine which species is oxidized and which is reduced:
1. Assign oxidation numbers to all atoms in each reactant and each product using the rules above.
2. Compare the oxidation numbers of each element that appears on both sides of the equation.
○ If an element’s oxidation number increases from reactant to product, that element is oxidized (it has lost electrons).
○ If an element’s oxidation number decreases from reactant to product, that element is reduced (it has gained electrons).
3. Identify the half‐reactions: separate the oxidation half‐reaction and the reduction half‐reaction. Balance each half‐reaction for mass and charge.
Detailed Explanation
This chunk outlines a systematic approach to identify which species gets oxidized and which gets reduced in a chemical reaction. By first assigning oxidation numbers, one can directly observe any changes. If a reactant's oxidation number increases, it has lost electrons and is oxidized, while a decrease indicates reduction. After determining these changes, the reaction can be broken into half-reactions—one for oxidation and one for reduction. Each half-reaction is then balanced to reflect the mass and charge accurately, which is vital for understanding electron flow in the reaction.
Examples & Analogies
Imagine a scoring system during a sports game. Each player's score at the beginning (oxidation number) can change throughout the game. When a player scores a point (gains electrons), their score increases (oxidation number increases), indicating 'oxidation.' Conversely, if they lose a point (lose electrons), their score decreases (oxidation number decreases), indicating 'reduction.' By tracking these changes, just as in a game, one can determine who is winning (oxidized) and who is losing (reduced) in the chemical reaction.
Balancing Redox Equations
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Balancing redox equations typically follows these steps:
1. Separate the reaction into two half‐reactions: one for oxidation, one for reduction.
2. Balance all atoms except hydrogen and oxygen in each half‐reaction.
3. Balance oxygen atoms by adding H2O molecules to whichever side lacks oxygen.
4. Balance hydrogen atoms by adding H+ ions (in acidic solution) to whichever side lacks hydrogen.
5. Balance charge by adding electrons to the more positive side until the total charges on both sides match.
Detailed Explanation
Here, we break down the method for balancing redox equations, essential for accurately representing these types of reactions. First, separate the redox reaction into its oxidation and reduction half-reactions. Then, ensure that all atoms are balanced, except for hydrogen and oxygen. Next, use water (H₂O) molecules to adjust oxygen amounts, and add H⁺ ions to balance any hydrogen discrepancies in acidic conditions. Lastly, adjust the electrons to equalize charge on both sides of the equation, ensuring a balanced redox equation represents both mass and charge conservation.
Examples & Analogies
Think of balancing a recipe where you need to have the same number of ingredients (atoms) on both sides. If you’re making a cake, you want the exact amount of flour (atoms) going in as you have coming out as cake. If you realize you have more sugar (an element) or need to adjust how many eggs (H⁺ ions), you add ingredients accordingly to ensure it's balanced. Just as recipes need balance to work, redox equations require a structured approach to balance all elements and charges.
Key Concepts
-
Oxidation: Refers to the loss of electrons and an increase in oxidation number.
-
Reduction: Refers to the gain of electrons and a decrease in oxidation number.
-
Oxidation Numbers: They serve as a tool for tracking electron transfer in reactions.
-
Balancing: The method of ensuring the total number of atoms and charge is the same on both sides of a reaction.
Examples & Applications
Zinc reacting with copper sulfate where zinc is oxidized to zinc ions, and copper(II) ions are reduced to copper metal.
Combustion of propane (C3H8) producing carbon dioxide and water, exemplifying a common combustion redox reaction.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When electrons go away, oxidation is at play!
Stories
Once upon a time, there was a brave zinc atom that lost its electrons to help copper make a new home, thus becoming a zinc ion.
Memory Tools
LEO says GER: Lose Electrons = Oxidation; Gain Electrons = Reduction.
Acronyms
OIL RIG
Oxidation Is Loss
Reduction Is Gain.
Flash Cards
Glossary
- Oxidation
The process of losing electrons, resulting in an increase in oxidation number.
- Reduction
The process of gaining electrons, leading to a decrease in oxidation number.
- Oxidation Numbers
Assigned integers representing an atom’s hypothetical charge in a compound.
- Redox Reaction
A chemical reaction involving the transfer of electrons between two species.
- HalfReaction
One part of a redox reaction, showing either oxidation or reduction separately.
- Balancing
The process of ensuring that mass and charge are the same on both sides of a chemical equation.
- Combustion
A rapid exothermic reaction of a fuel with oxygen resulting in heat and light.
- Corrosion
The degradation of metals due to a reaction with environmental elements.
Reference links
Supplementary resources to enhance your learning experience.
