Energetics/Thermochemistry
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Enthalpy Changes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome, class! Today we're diving into energetics, specifically the changes in energy during chemical reactions, which we measure as enthalpy changes. Who can tell me what enthalpy is?

Is it the total heat content of a system?

Exactly! It's expressed as 'H' and measured at constant pressure. Now, can anyone explain the difference between exothermic and endothermic reactions?

Exothermic reactions release heat, while endothermic reactions absorb heat!

Right! And this is quantified as the enthalpy change, ΔH. What do you think a negative ΔH signifies?

It means the reaction is exothermic!

That's correct! Please remember the acronym 'E for Exit' to associate exothermic with heat leaving.

What about the endothermic reactions, then?

Great question! An endothermic reaction has a positive ΔH, indicating it absorbs heat. So, think 'E for Enter'—heat enters the system.

To sum it up: exothermic reactions discharge heat (ΔH < 0), and endothermic reactions intake heat (ΔH > 0).
Standard Enthalpy Changes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s focus now on specific types of standard enthalpy changes. Can someone define the standard enthalpy of formation?

It’s the heat change when one mole of a compound forms from its elements in their standard states.

Right! For example, the formation of CO₂ has a ΔH_f° of -393.5 kJ mol⁻¹. Why do you think the ΔH_f° of oxygen gas is zero?

Because it's in its standard state?

Exactly! It’s the baseline reference. Now, who can explain the standard enthalpy of combustion?

It's the heat change when one mole of a substance completely combusts in oxygen.

Spot on! And remember, combustion reactions are always exothermic, hence ΔH_c° values are negative.

What about neutralization reactions?

Neutralization is the reaction of an acid with a base to produce water and salt, typically with a ΔH_neut° of around -57.3 kJ mol⁻¹ for strong acid-base reactions.

Let’s remember the acronym 'NEUTRAL' to relate it to neutralization reactions!
Calorimetry and Measurement of Enthalpy Changes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s discuss how we measure these enthalpy changes. Who knows about calorimetry?

It’s an experimental method to measure heat changes, right?

Exactly. We often use simple calorimeters, like polystyrene cups. The formula we use is `q = mcΔT`. Who can explain these variables?

q is heat exchanged, m is the mass, c is specific heat capacity, and ΔT is the change in temperature.

Good job! After calculating q, how do we relate this back to ΔH?

We divide q by the number of moles of the reactants.

Yes! It's also crucial to consider the signs: positive q means an exothermic reaction, but ΔH for the reaction is negative.

And vice versa for endothermic reactions, right?

Exactly! Remember: ‘Heat in is positive, heat out is negative’ for a quick reminder!
Understanding Hess's Law
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's shift gears and discuss Hess's Law. Does anyone remember what this law states?

It says that the total enthalpy change for a reaction is equal to the sum of enthalpy changes of individual steps.

Correct! It’s a critical tool when reactions can’t be directly measured. Can anyone provide an example of how we might use Hess's Law?

We could use standard enthalpies of formation to calculate the reaction’s total enthalpy change.

Exactly! Remember the formula: ΔH_rxn° = ΣnΔH_f°(products) - ΣmΔH_f°(reactants). What happens if we reverse the reaction?

The sign of ΔH changes as well!

Perfect! And if we multiply an equation by a factor, what happens to ΔH?

It gets multiplied by the same factor!

Exactly! Understanding Hess's Law is key to calculating enthalpy changes effectively.
Bond Enthalpies
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s talk about bond enthalpies. What do we mean by bond enthalpy?

It's the energy required to break one mole of a specific type of bond.

Correct! And how do we estimate the enthalpy change using bond enthalpies?

We sum the bond enthalpies of bonds broken and subtract the bond enthalpies of bonds formed.

Exactly! Remember, these values are averages, which is why they're estimations. Can anyone give an example of how we could calculate ΔH using bond enthalpies?

Like for the combustion of methane, we'd list the bonds broken and formed and plug those into the calculation!

Perfectly put! Always remember: bond breaking is endothermic, and bond formation is exothermic!

So, we add the energy of bonds broken and then subtract the energy of bonds formed to get ΔH!

That's right! Keep practicing with these calculations, and it’ll become second nature.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section breaks down the concepts of enthalpy changes (ΔH) associated with chemical processes, outlining key principles such as standard enthalpy of formation, combustion, and neutralization. It also addresses Hess's Law, bond enthalpies, and the role of Gibbs Free Energy and entropy in predicting reaction spontaneity.
Detailed
Energetics/Thermochemistry
Energetics, also known as thermochemistry, focuses on the energy changes that occur during chemical and physical processes. Energy can be absorbed (endothermic reactions) or released (exothermic reactions) in chemical reactions, typically measured as enthalpy change (ΔH). Enthalpy (H) reflects the total heat content of a system at constant pressure and is a state function.
4.1 Enthalpy Changes
Enthalpy changes describe the heat exchange in reactions, categorized as:
- Exothermic reactions: Release heat (ΔH < 0, e.g., combustion, neutralization).
- Endothermic reactions: Absorb heat (ΔH > 0, e.g., melting ice, photosynthesis).
Standard conditions for measuring enthalpy changes are defined (1 atm, 25 °C). Key standard enthalpy changes include:
- Standard Enthalpy of Formation (ΔH_f°): Formation of a compound from its elements (e.g., ΔH_f°(O₂(g)) = 0).
- Standard Enthalpy of Combustion (ΔH_c°): Heat change during the combustion of a substance (always negative).
- Standard Enthalpy of Neutralization (ΔH_neut°): Heat change during neutralization (approximately -57.3 kJ mol⁻¹ for strong acids and bases).
Measurement (Calorimetry)
Enthalpy changes are measured using calorimetry, determining heat exchanged (q) using the formula q = mcΔT. The calculated enthalpy change is adjusted for the reaction's stoichiometry and heat direction.
4.2 Hess's Law
Hess's Law states that the total enthalpy change in a reaction is equal to the sum of the enthalpy changes of individual steps. This principle is crucial for calculating enthalpy changes of multi-step reactions using known enthalpy changes. Applications include:
- Using standard enthalpies of formation.
- Manipulating equations to match target equations for calculation.
4.3 Bond Enthalpies
Bond enthalpy measures energy required to break a bond. The overall change in a reaction's enthalpy can be estimated by calculating the difference between energy used to break bonds and energy released forming bonds, using average bond enthalpy values.
4.4 HL: Entropy and Gibbs Free Energy
While ΔH indicates heat change, entropy (S) measures system disorder. Gibbs Free Energy (G) combines enthalpy and entropy to predict spontaneity:
ΔG = ΔH - TΔS.
Negative ΔG indicates spontaneous reactions, while positive ΔG suggests non-spontaneity.
4.5 HL: Spontaneity of Reactions
The interplay of ΔH and ΔS determines reaction spontaneity influenced by temperature. The equilibrium temperature (T_eq) at which ΔG = 0 is key for understanding reaction favorability. This framework is essential for practical chemistry applications, highlighting energy and disorder dynamics in chemical systems.
Youtube Videos

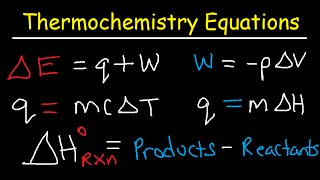

Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Energetics and Thermochemistry
Chapter 1 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Energetics, or thermochemistry, is the study of energy changes that accompany chemical and physical processes. All chemical reactions involve energy changes, either absorbing energy from the surroundings (endothermic) or releasing energy to the surroundings (exothermic). This energy is typically exchanged as heat and is quantified as an enthalpy change (ΔH).
Detailed Explanation
Energetics or thermochemistry focuses on how energy changes during chemical reactions and physical processes. Reactions can absorb heat (endothermic), making them feel cold, or release heat (exothermic), making them feel hot. The change in energy during a reaction is measured by enthalpy change (ΔH), which tells us how much energy is absorbed or released.
Examples & Analogies
Think of cooking pasta. When you boil water (an exothermic process), it releases heat into the air. If you put pasta into the boiling water, the pasta absorbs some heat (endothermic), causing it to cook.
Understanding Enthalpy (H)
Chapter 2 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Enthalpy (H) is a thermodynamic property that represents the total heat content of a system at constant pressure. It is a state function, meaning its value depends only on the initial and final states of the system, not on the path taken.
Detailed Explanation
Enthalpy (H) measures the total heat content of a system when the pressure remains constant. It’s considered a state function because it does not depend on how the system got from its initial state to its final state, just on the state itself. For example, whether you heat water slowly or quickly to boil it, the enthalpy change will be the same.
Examples & Analogies
Imagine a journey from home to school. It doesn't matter how you get there (walking, driving, biking), the distance you traveled remains the same. Similarly, enthalpy changes focus only on the start and end points of a reaction.
Enthalpy Changes in Reactions
Chapter 3 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Enthalpy change (ΔH) is the heat absorbed or released during a chemical reaction at constant pressure.
- Exothermic reactions: Release heat to the surroundings. The enthalpy of the products is lower than the enthalpy of the reactants, so ΔH is negative (ΔH < 0).
- Endothermic reactions: Absorb heat from the surroundings. The enthalpy of the products is higher than the enthalpy of the reactants, so ΔH is positive (ΔH > 0).
Detailed Explanation
ΔH indicates the heat transfer during a chemical reaction at constant pressure. In exothermic reactions, heat is released, resulting in a negative change in enthalpy (ΔH < 0). Conversely, endothermic reactions absorb heat, leading to a positive enthalpy change (ΔH > 0). This indicates how heat impacts the products compared to the reactants.
Examples & Analogies
Consider a fire burning wood (exothermic). It releases heat, warming up the room. Now think about melting ice (endothermic). It requires heat from the environment to change from solid to liquid, making the surrounding air feel cooler.
Standard Conditions for Measuring Enthalpy Changes
Chapter 4 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Enthalpy changes are typically measured under standard conditions to allow for comparison. Standard conditions are defined as:
- Pressure: 100 kPa (1 atm)
- Temperature: 298 K (25 °C)
- Concentration: 1 mol dm⁻³ for solutions
A superscript circle (°) is used to denote standard conditions (e.g., ΔH°).
Detailed Explanation
To ensure consistency and comparability in measuring enthalpy changes, experiments are conducted under standard conditions—specific temperature, pressure, and concentration. These conditions allow scientists to report and compare results effectively.
Examples & Analogies
It's like baking a cake. Following a standard recipe with set measurements (like 350°F for the oven) ensures your cake turns out well every time. Standard conditions in experiments help ensure reliable and reproducible results.
Standard Enthalpy of Formation (ΔH_f°)
Chapter 5 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The standard enthalpy of formation of a compound is the enthalpy change when one mole of a compound is formed from its constituent elements in their standard states under standard conditions.
- By definition, the standard enthalpy of formation of an element in its most stable form under standard conditions is zero. For example, ΔH_f°(O₂(g)) = 0, ΔH_f°(C(graphite)) = 0.
- Example: C(graphite) + O₂(g) → CO₂(g) ΔH_f° = -393.5 kJ mol⁻¹ (for CO₂).
Detailed Explanation
The standard enthalpy of formation (ΔH_f°) measures how much energy is involved when a compound is formed from its basic elements in their most stable state. Elements in their standard form have an enthalpy of formation of zero. Understanding this concept helps in calculating the energy changes when forming various compounds.
Examples & Analogies
Think of assembling furniture from its individual pieces. When you put everything together (forming the product), the energy used is akin to the enthalpy of formation. In a sense, you're measuring the 'effort' it takes to complete the assembly.
Standard Enthalpy of Combustion (ΔH_c°)
Chapter 6 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The standard enthalpy of combustion of a substance is the enthalpy change when one mole of a substance undergoes complete combustion in excess oxygen under standard conditions.
- Combustion reactions are always exothermic, so ΔH_c° values are always negative.
- Example: CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l) ΔH_c° = -890.3 kJ mol⁻¹ (for CH₄).
Detailed Explanation
The standard enthalpy of combustion (ΔH_c°) measures the energy released when a substance completely burns in oxygen. Since these reactions release heat, their enthalpy values are negative. Knowing this helps predict how much energy one can get from burning a fuel.
Examples & Analogies
Consider an outdoor barbecue. When you light the charcoal (burning it), it releases heat that cooks your food. The energy released during that combustion is what we're quantifying with ΔH_c°.
Standard Enthalpy of Neutralization (ΔH_neut°)
Chapter 7 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The standard enthalpy of neutralization is the enthalpy change when one mole of water is formed from the reaction of an acid and a base under standard conditions. For strong acid-strong base reactions, the enthalpy of neutralization is remarkably consistent, approximately -57.3 kJ mol⁻¹, because the net ionic equation is always the same: H⁺(aq) + OH⁻(aq) → H₂O(l).
- Example: HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l) ΔH_neut° ≈ -57.3 kJ mol⁻¹.
Detailed Explanation
The standard enthalpy of neutralization measures the heat released when an acid reacts with a base to form water. This value is consistent across strong acid-base reactions because the same ions (H⁺ and OH⁻) recombine to form water. Understanding this helps predict the heat produced during neutralization reactions in various chemical processes.
Examples & Analogies
Mixing vinegar (acid) and baking soda (base) produces bubbles and heat—like a mini-explosion! This reaction exemplifies the concept of neutralization, showcasing how energy is released when acetic acid and sodium bicarbonate react.
Measurement of Enthalpy Changes (Calorimetry)
Chapter 8 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Enthalpy changes are measured experimentally using a calorimeter. A simple calorimeter can be a polystyrene cup or a bomb calorimeter for more accurate measurements. The principle involves measuring the temperature change (ΔT) of a known mass of water (or solution) due to the heat released or absorbed by a reaction.
The heat exchanged (q) is calculated using the formula: q = mcΔT
Where:
- q = heat energy transferred (Joules, J)
- m = mass of the substance (water/solution) that changes temperature (grams, g)
- c = specific heat capacity of the substance (for water, 4.18 J g⁻¹ K⁻¹ or J g⁻¹ °C⁻¹)
- ΔT = change in temperature (K or °C)
Once 'q' is determined, the enthalpy change (ΔH) for the reaction can be calculated by dividing 'q' by the number of moles of reactant that caused that heat change. Remember to consider the sign convention: if the solution temperature increases (exothermic reaction), q is positive for the surroundings, but ΔH for the reaction is negative. If the solution temperature decreases (endothermic reaction), q is negative for the surroundings, and ΔH for the reaction is positive.
Detailed Explanation
Calorimetry is the experimental technique for measuring the heat changes in reactions. Using a calorimeter, we monitor the temperature change of a liquid (often water) that absorbs or releases heat due to the reaction. We can calculate the heat transfer (q) using a specific formula, accounting for the mass of the liquid, its specific heat capacity, and the temperature change to determine the enthalpy change (ΔH). This process shows how reactions impact their surroundings.
Examples & Analogies
Imagine putting a thermometer in a hot cup of coffee. As the coffee cools off, the temperature reading goes down. That temperature change reflects the heat lost to the surrounding air. A calorimeter works similarly to measure heat exchanges in chemical reactions.
Key Concepts
-
Thermochemistry: The study of energy changes during chemical and physical processes.
-
Enthalpy (H): A state function representing total heat content, varying with initial and final states.
-
Exothermic and Endothermic Reactions: Release or absorb heat, respectively; indicated by the sign of ΔH.
-
Standard Conditions: Defined for reproducibility in measuring enthalpy changes.
-
Hess's Law: A principle for calculating enthalpy changes by summing individual step changes.
-
Bond Enthalpy: Energy needed to break specific bonds, averaged for calculation purposes.
-
Entropy (S): A measure of disorder, influencing spontaneity of reactions alongside Gibbs Free Energy.
-
Gibbs Free Energy (G): Combines enthalpy and entropy to predict reaction feasibility.
Examples & Applications
Combustion of methane: CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l) with ΔH_c° = -890.3 kJ mol⁻¹.
Neutralization of hydrochloric acid with sodium hydroxide: HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l) with ΔH_neut° ≈ -57.3 kJ mol⁻¹.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Exothermic heat flies away, endothermic heat comes to stay.
Stories
Imagine a pot on a stove: when you heat it, steam flies out (exothermic), but when you place ice in it, it absorbs heat to melt (endothermic).
Memory Tools
Remember 'HESS' for Hess's Law: 'H' for heat, 'E' for equal, 'S' for steps, 'S' for sum total.
Acronyms
Use 'BEAT' to remember bond enthalpy
'B' for bonds broken
'E' for energy
'A' for average
'T' for total energy calculation.
Flash Cards
Glossary
- Enthalpy (H)
A thermodynamic property representing the total heat content of a system at constant pressure.
- Enthalpy Change (ΔH)
The heat absorbed or released during a chemical reaction at constant pressure.
- Exothermic Reaction
A reaction that releases heat to the surroundings, indicated by a negative ΔH (ΔH < 0).
- Endothermic Reaction
A reaction that absorbs heat from the surroundings, indicated by a positive ΔH (ΔH > 0).
- Standard Enthalpy of Formation (ΔH_f°)
The heat change when one mole of a compound is formed from its elements in their standard states.
- Standard Enthalpy of Combustion (ΔH_c°)
The heat change during the complete combustion of one mole of a substance in excess oxygen.
- Standard Enthalpy of Neutralization (ΔH_neut°)
The enthalpy change when one mole of water is formed from an acid-base reaction.
- Calorimetry
A method to measure the heat changes in a reaction using calorimeters.
- Hess's Law
The principle stating that the total enthalpy change for a reaction is equal to the sum of the enthalpy changes of the individual steps.
- Bond Enthalpy
The energy required to break one mole of a specific type of bond in the gaseous state.
Reference links
Supplementary resources to enhance your learning experience.
