Hess's Law
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Hess's Law
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will explore Hess's Law, an important principle in thermochemistry. Can anyone tell me what Hess's Law states?

I think it relates to how we calculate heat changes in reactions, but I'm not sure how.

That's a great start! Hess's Law states that if a reaction can occur in a series of steps, the overall enthalpy change for the reaction is the sum of the enthalpy changes for each individual step. What do you think this means about the pathway taken by the reaction?

It sounds like the pathway doesn't matter as long as we know the individual steps.

Exactly! Since enthalpy is a state function, it only depends on the initial and final states of the system. Let's remember this with the acronym 'PATH' – it’s the *P*roducts *A*lways denotes *T*hermal *H*istory!

I like that! So, we can work out the total change even if we can't measure it directly?

Yes! That's the power of Hess's Law. Now, let’s move on to the manipulation of chemical equations.
Manipulating Equations
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To apply Hess's Law, we often need to manipulate our equations. Who can tell me what happens if we reverse a chemical equation?

The sign of the enthalpy change reverses too!

Right! For example, if we have A → B with ΔH = ΔH₁, reversing gives B → A with ΔH = -ΔH₁. Can anyone think of a scenario when we might need to reverse an equation?

Maybe when we know the products but not the reactants?

Exactly! And what about when we multiply a balanced equation by a factor?

The enthalpy change has to be multiplied by that same factor too.

Correct! Let’s remember this with the mnemonic ‘RAMP’ - *R*everse means *A*bsolute shift, *M*ultiply enthalpy and increase its *P*ower!
Applications of Hess's Law
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s talk about some applications of Hess's Law. What is one common way to apply it?

Using standard enthalpies of formation to find the ΔH for a reaction!

Absolutely! The formula is ΔH_rxn° = ΣnΔH_f°(products) - ΣmΔH_f°(reactants). Can someone break this down for me?

We add up the enthalpy changes of the products and subtract those of the reactants.

Spot on! Now let’s take a practical example, such as the combustion of methane. How would we apply Hess's Law here?

We would find the enthalpy changes for CO₂ and H₂O, then subtract the one for CH₄.

Yes! Now, let’s remember this with another acronym, ‘FIND’, which stands for *F*ind individual ΔH_f° values, *I*nput to the equation, *N*eglect lumps, and *D*eliver the ΔH!
Example Problem Solving
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s put what we learned into practice. Here's a reaction: C + ½O₂ → CO. We know two reactions with their ΔH values. How do we derive the enthalpy change?

We should keep the first equation and reverse the second one, changing its sign!

Perfect! Now, if our first reaction has ΔH₁ = -393.5 kJ and the second one is CO + ½O₂ → CO₂ with ΔH₂ = -283.0 kJ, how do we combine them?

We add ΔH₁ + (-ΔH₂)?

Exactly! For a net reaction, what do we get?

ΔH = -393.5 + 283.0, which gives us -110.5 kJ!

Great job! This application shows how powerful Hess's Law is for complex calculations.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Hess's Law is a crucial concept in thermochemistry, indicating that if a chemical reaction occurs in multiple steps, the total enthalpy change can be calculated by summing the enthalpy changes for each step. This principle allows us to determine enthalpy changes for reactions that are difficult to measure directly in a calorimeter.
Detailed
Hess's Law
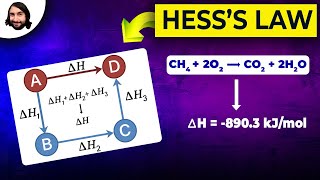
Hess's Law, or the Law of Constant Heat Summation, provides a method for calculating enthalpy changes in chemical reactions that occur in multiple steps or cannot be measured directly. According to Hess's Law, the overall enthalpy change for a reaction is equal to the sum of the enthalpy changes for each individual step in the reaction, an important outcome of the fact that enthalpy is a state function. This leads to two main manipulations of equations: 1) reversing a reaction changes the sign of the enthalpy change; and 2) multiplying the coefficients in a balanced equation also requires corresponding adjustments to the enthalpy change.
Applications of Hess's Law include:
1. Calculating standard enthalpy changes of reactions using standard enthalpies of formation.
2. Using a series of known reactions to derive the enthalpy of a target reaction through algebraic manipulation of the given equations.
Overall, Hess's Law is vital for solving complex enthalpy calculations and supports the concept of enthalpy as a state function.
Youtube Videos



Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Hess's Law
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Many chemical reactions are difficult or impossible to measure directly in a calorimeter. For such reactions, or for calculating enthalpy changes of reactions that proceed through multiple steps, Hess's Law of Constant Heat Summation is invaluable.
Hess's Law states that if a reaction can occur in a series of steps, the overall enthalpy change for the reaction is equal to the sum of the enthalpy changes for each individual step, regardless of the pathway taken. This is a direct consequence of enthalpy being a state function.
Detailed Explanation
Hess's Law helps us determine the heat change (enthalpy change) in chemical reactions that are hard to measure directly. It tells us that if a reaction can happen in several stages, the total heat change when going from reactants to products is the same, no matter how many steps are involved. This is because enthalpy is a property that only depends on the initial and final states of the system, not the steps taken to get there.
Examples & Analogies
Think of Hess's Law like taking a road trip. If you take a series of different routes to get to your destination, it doesn't change the total distance you’ve traveled. Whether you took one long road or several smaller roads, the total distance remains the same, just like the total heat change remains the same regardless of the pathway in a chemical reaction.
Manipulating Equations and Enthalpy Changes
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Essentially, if you can manipulate known chemical equations and their enthalpy changes to sum up to a target equation, then the sum of their enthalpy changes will give you the enthalpy change of the target equation.
- Reversing an equation: If a reaction is reversed, the sign of its enthalpy change must also be reversed.
- A → B ΔH₁
- B → A ΔH = -ΔH₁
- Multiplying an equation by a factor: If the stoichiometric coefficients in an equation are multiplied by a factor, the enthalpy change must also be multiplied by the same factor.
- A → B ΔH₁
- 2A → 2B ΔH = 2ΔH₁
Detailed Explanation
To apply Hess’s Law effectively, we often need to rearrange known chemical equations. If you reverse an equation, you not only change the direction of the reaction, but you must also change the sign of the enthalpy. For example, if a process releases energy (negative ΔH), reversing it means it will now absorb energy (positive ΔH). Similarly, if you double the amounts of substances in a chemical equation, you must also double the enthalpy change. These manipulations allow you to align known reactions to derive the enthalpy change for a new reaction.
Examples & Analogies
Imagine baking a cake by following a recipe. If you want to make a smaller cake, you can either reverse the process by removing ingredients or scale down the recipe. If you remove an ingredient, you would need to adjust all other ingredients to maintain the correct proportions. Similarly, in Hess's Law, any change to the stoichiometric coefficients must also affect the enthalpy change correspondingly.
Applications of Hess's Law
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Hess's Law is commonly used in two main ways:
1. Using Standard Enthalpies of Formation (ΔH_f°): The standard enthalpy change of a reaction (ΔH_rxn°) can be calculated from the standard enthalpies of formation of the products and reactants using the formula:
ΔH_rxn° = ΣnΔH_f°(products) - ΣmΔH_f°(reactants)
Where 'n' and 'm' are the stoichiometric coefficients in the balanced chemical equation. Remember that the ΔH_f° for elements in their standard states is zero.
Example: Calculate ΔH_rxn° for the combustion of methane: CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l)
Given: ΔH_f°(CH₄(g)) = -74.8 kJ mol⁻¹ ΔH_f°(CO₂(g)) = -393.5 kJ mol⁻¹ ΔH_f°(H₂O(l)) = -285.8 kJ mol⁻¹ ΔH_f°(O₂(g)) = 0 kJ mol⁻¹
ΔH_rxn° = [1 × ΔH_f°(CO₂(g)) + 2 × ΔH_f°(H₂O(l))] - [1 × ΔH_f°(CH₄(g)) + 2 × ΔH_f°(O₂(g))]
ΔH_rxn° = [1 × (-393.5) + 2 × (-285.8)] - [1 × (-74.8) + 2 × (0)] ΔH_rxn° = [-393.5 - 571.6] - [-74.8] ΔH_rxn° = -965.1 + 74.8 = -890.3 kJ mol⁻¹
- Using a series of known reactions: This involves algebraically manipulating given equations to match the target equation.
Detailed Explanation
Hess's Law is applied mainly in two ways. First, to calculate the enthalpy change of a reaction using standard enthalpies of formation. The formula takes into account the enthalpy changes of products and reactants, which allows us to determine the overall change for the reaction. Second, it enables us to use known reactions and manipulate their equations to derive the enthalpy change for a new target reaction. This algebraic manipulation is key to calculating enthalpy changes for reactions that are difficult to measure directly.
Examples & Analogies
Think of solving a puzzle where you know some pieces (reactions) can be combined to create a larger picture (target reaction). By carefully selecting and connecting the right pieces together, just like adding the enthalpies from each piece, you can discover the total enthalpy change for the complete picture. The process is similar to following a known recipe, where known ingredients lead to desired outcomes.
Key Concepts
-
Hess's Law: The overall enthalpy change is the sum of the individual steps.
-
Enthalpy Change (ΔH): Represents heat absorbed or released in a reaction.
-
Reversing Equations: Changing the sign of ΔH when a reaction is reversed.
-
Multiplying Equations: Multiplying the reaction coefficients also scales ΔH.
Examples & Applications
Calculating the ΔH for combustion reactions using standard enthalpy of formation.
Using known reactions to derive ΔH for a target reaction via manipulation of equations.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Hess's law, oh what a joy, sum the steps, don’t be coy!
Stories
Imagine you're on a journey with different paths. No matter how you choose to go, the distance doesn't change!
Memory Tools
Remember ‘RAMP’ for manipulating: Reverse, Absolute, Multiply, Power!
Acronyms
Use ‘FIND’ for enthalpy changes
*F*ind values
*I*nput them
*N*eglect lumps
*D*eliver ΔH.
Flash Cards
Glossary
- Hess's Law
The principle stating that the total enthalpy change of a reaction is the sum of the enthalpy changes for each step.
- Enthalpy Change (ΔH)
The heat absorbed or released during a chemical reaction at constant pressure.
- Standard Enthalpy of Formation (ΔH_f°)
The enthalpy change when one mole of a compound is formed from its elements in their standard states.
- Exothermic Reaction
A reaction that releases heat, resulting in a negative ΔH.
- Endothermic Reaction
A reaction that absorbs heat, resulting in a positive ΔH.
Reference links
Supplementary resources to enhance your learning experience.
