Manufacture – Contact Process
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to the Contact Process
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will discuss the Contact Process, which is a method used for manufacturing sulfuric acid. Let's start with the first step: burning sulfur.

What happens when we burn sulfur?

Great question! Burning sulfur reacts with oxygen to form sulfur dioxide. So, we can remember this as 'SO₂ from burning sulfur'.

Is burning the only way to get SO₂?

Good point! We can also derive SO₂ from sulfide ores. But for the Contact Process, we primarily focus on burning.
Oxidation of SO₂ to SO₃
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Once we have sulfur dioxide, we need to convert it to sulfur trioxide using a catalyst. This is where vanadium pentoxide comes in.

What is a catalyst?

A catalyst is a substance that speeds up a reaction without being consumed. So, remember: 'V₂O₅ for the victory in oxidation!'

How do we write the reaction for this step?

We can write it as: 2SO₂ + O₂ ⟶ 2SO₃. Remember this equation.
Absorption and Dilution Steps
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, we need to absorb the sulfur trioxide in concentrated sulfuric acid to form oleum. Does anyone know what oleum is?

Is it a special type of sulfuric acid?

Exactly! It's a solution of SO₃ in H₂SO₄. Finally, add water to dilute oleum to obtain sulfuric acid. We say: 'Add water carefully to avoid splashes!'

So, the final product is H₂SO₄?

Yes! And that's the complete process to manufacture sulfuric acid. Let's recap: Burn sulfur, oxidize to SO₃, absorb in H₂SO₄, and dilute!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
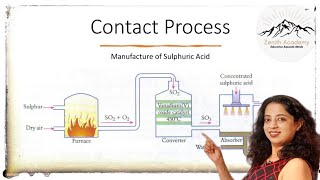
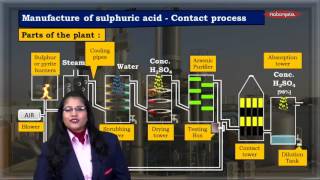
This section outlines the steps involved in the Contact Process for manufacturing sulfuric acid, starting with the burning of sulfur or sulfide ores to form sulfur dioxide, its subsequent oxidation to sulfur trioxide, absorption in concentrated sulfuric acid, and finally dilution to produce sulfuric acid.
Detailed
The Contact Process involves four primary steps in the manufacture of sulfuric acid:
- Burning of Sulfur: Sulfur or sulfide ores are combusted to produce sulfur dioxide (SO₂).
- Oxidation of SO₂: Using a vanadium pentoxide (V₂O₅) catalyst, sulfur dioxide is oxidized to sulfur trioxide (SO₃).
2SO₂ + O₂ ⟶ 2SO₃ (with V₂O₅ catalyst)
- Absorption of SO₃: The sulfur trioxide is then absorbed in concentrated sulfuric acid to form oleum.
- Dilution with Water: Finally, oleum is diluted with water to yield sulfuric acid (H₂SO₄).
The Contact Process is a significant industrial method for producing H₂SO₄, which is crucial in various chemical processes and industries.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Burning Sulfur
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Burning sulfur or sulfide ores to form SO₂
Detailed Explanation
The first step in the Contact Process for manufacturing sulfuric acid involves the combustion of sulfur or sulfide ores. In this step, sulfur (S) reacts with oxygen (O₂) from the air to produce sulfur dioxide (SO₂). This chemical reaction can be simplified as:
S + O₂ → SO₂
This reaction is essential because sulfur dioxide is a key intermediate in the production of sulfuric acid.
Examples & Analogies
Think of this process like starting a campfire. Just as you need wood (sulfur) and oxygen (air) to ignite a flame (SO₂), sulfur needs oxygen to burn and form sulfur dioxide, which starts the process of creating something more complex.
Oxidation of SO₂
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Oxidation of SO₂ to SO₃ using V₂O₅ catalyst:
2SO₂ + O₂ → V₂O₅ 2SO₃
Detailed Explanation
After sulfur dioxide (SO₂) is produced, the next step is the oxidation of SO₂ to sulfur trioxide (SO₃). This occurs when sulfur dioxide gas reacts with more oxygen in the presence of a vanadium pentoxide (V₂O₅) catalyst. The catalyst accelerates the reaction without being consumed. The reaction can be summarized as:
2SO₂ + O₂ → 2SO₃
This step is critical because sulfur trioxide is the compound that, when dissolved, leads to the formation of sulfuric acid.
Examples & Analogies
Imagine riding a bicycle on a small hill (the V₂O₅ catalyst). You need some initial push (the oxygen) to get to the top (the SO₃ production). The hill makes it easier for you to reach your destination without needing to exert too much energy – that's what the catalyst does in this process.
Absorption of SO₃
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Absorption of SO₃ in concentrated H₂SO₄ to form oleum
Detailed Explanation
The next step in the Contact Process involves the absorption of sulfur trioxide (SO₃) in concentrated sulfuric acid (H₂SO₄). When SO₃ dissolves in H₂SO₄, it forms a compound known as oleum (H₂S₂O₇). This interaction results in a higher concentration of sulfuric acid and can be represented as:
SO₃ + H₂SO₄ → H₂S₂O₇ (oleum)
This step is essential because oleum can be further diluted to produce sulfuric acid.
Examples & Analogies
Think of this absorption process like a sponge soaking up water. The sponge (H₂SO₄) can absorb a substantial amount of water (SO₃) to become heavier and more saturated (forming oleum). Just as a sponge can retain moisture, concentrated sulfuric acid can retain and react with sulfur trioxide.
Dilution of Oleum
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Dilution of oleum with water to get H₂SO₄
Detailed Explanation
The final step in the Contact Process is the dilution of oleum with water to produce sulfuric acid (H₂SO₄). When oleum is mixed with water, it reacts to form sulfuric acid, which is a highly concentrated and useful product. The reaction can be summarized as:
H₂S₂O₇ + H₂O → 2H₂SO₄
This step transforms the oleum into sulfuric acid, ready for various applications.
Examples & Analogies
This dilution process can be likened to making lemonade. When you have a concentrated lemon juice (oleum) and you add water, the drink becomes enjoyable and palatable (diluted sulfuric acid). Just like too much lemon juice without dilution would be overwhelming, oleum must be diluted to create useful sulfuric acid.
Key Concepts
-
Contact Process: A method for producing sulfuric acid involving multiple steps.
-
Oxidation: The conversion of sulfur dioxide to sulfur trioxide using a catalyst.
-
Sulfur Trioxide: A key intermediate product in the manufacture of sulfuric acid.
Examples & Applications
The reaction 2SO₂ + O₂ ⟶ 2SO₃ represents the oxidation of sulfur dioxide to sulfur trioxide.
Absorbing SO₃ in concentrated H₂SO₄ to form oleum demonstrates the synthesis of oleum.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Burn sulfur bright, to SO₂ take flight, Oxidize with V₂O₅, make SO₃ ignite!
Stories
Once upon a time, sulfur burned and turned into a gas, SO₂. Then, with the help of a magical catalyst, it transformed into SO₃. The wise chemist absorbed SO₃ in sulfuric acid, creating oleum, and finally added water to make the potent H₂SO₄.
Memory Tools
B.O.A.D: Burn, Oxidize, Absorb, Dilute.
Acronyms
SOAP - Sulfur, Oxidation, Absorption, Production for Contact Process.
Flash Cards
Glossary
- Contact Process
An industrial method for producing sulfuric acid by oxidizing sulfur dioxide to sulfur trioxide and absorbing it in sulfuric acid.
- Vanadium Pentoxide (V₂O₅)
A catalyst used to speed up the oxidation of sulfur dioxide in the production of sulfur trioxide.
- Oleum
A solution of sulfur trioxide in concentrated sulfuric acid.
- Sulfur Dioxide (SO₂)
A gas produced by burning sulfur, which is converted to sulfur trioxide in the Contact Process.
- Sulfur Trioxide (SO₃)
A gas formed from the oxidation of sulfur dioxide, absorbed to produce oleum.
Reference links
Supplementary resources to enhance your learning experience.
