Ozone Layer and Its Depletion
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding the Ozone Layer
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’ll learn about the ozone layer, which is essential, as it absorbs harmful UV rays from the sun. Who can tell me why this is important?

It protects us from sunburn and skin cancer?

Exactly! Remember the acronym UV: U for 'Ultraviolet,' and V for 'Vulnerability' because without the ozone layer, we are vulnerable to these dangerous rays.

So, it’s like a shield for the Earth?

Yes! A protective shield is a great way to think about it. Any other thoughts on why the ozone layer is vital?

It helps keep ecosystems safe too, right?

Absolutely, ecosystems depend on the ozone layer for their health. It ensures that plants and aquatic life can thrive.

What happens if it gets depleted?

Good question! Let’s discuss that next.
Causes of Ozone Depletion
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s talk about what causes ozone depletion. The main culprits are substances called CFCs. Can anyone say what CFC stands for?

Chlorofluorocarbons?

Correct! CFCs are found in many household products like refrigerators and aerosol sprays. Why do you think they are harmful?

They break down ozone molecules?

Yes, they release chlorine when they reach the stratosphere, which then depletes ozone. A quick memory aid: think of 'CFC' as 'Caution: Freddy's Chlorine!' which symbolizes that CFCs bring a ‘caution’ to our protective layer.

What else can cause ozone depletion?

Other substances like halons and certain solvents also contribute. Let's keep these in mind as we discuss their impacts next.
Consequences of Ozone Layer Depletion
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we know the causes, let’s discuss the consequences. What do you think happens when the ozone layer thins out?

More sunburns and skin cancers?

Exactly! The risk of skin cancer increases due to more UV rays hitting the Earth. Remember the phrase, 'No Ozone, More Problems!'

And it damages crops too, right?

Right! It affects plant growth and marine life. The whole food chain and ecosystem can suffer. Can any of you think of ways we could mitigate this?

By using less CFCs or switching to safer alternatives?

Great answer! Reducing CFC usage is key in protecting our ozone layer and the planet.
Protecting the Ozone Layer
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To protect the ozone layer, we must take action. What do you think we should do?

Educate people about the harms of CFCs?

Exactly! Spreading awareness is crucial. Another great way is to support policies that reduce the use of these harmful substances. Remember: 'Choose wisely, protect the sky!'

Are there alternatives to CFCs?

Yes! Hydrofluorocarbons (HFCs) are being used instead, but we should also aim for more sustainable options. Let's conclude by summarizing what we've learned about the ozone layer and its importance.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The ozone layer acts as Earth's sunscreen by absorbing harmful UV radiation. Depletion of this layer, primarily caused by chlorofluorocarbons (CFCs) from everyday products like refrigerators and sprays, has far-reaching consequences, including increased skin cancer and damage to crops and marine ecosystems.
Detailed
Ozone Layer and Its Depletion
The ozone layer is a critical component of the Earth's atmosphere that absorbs most of the Sun's harmful ultraviolet (UV) radiation. Without this protective layer, life on Earth would face increased risks, including a significant rise in skin cancer rates among humans and detrimental effects on various ecosystems.
Causes of Ozone Depletion
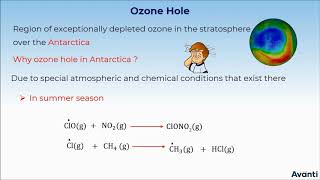
Ozone depletion is primarily attributed to chlorofluorocarbons (CFCs), which are released from a range of common sources such as refrigerators, air conditioning systems, spray aerosols, and foam-blowing agents. These substances contain chlorine and bromine — elements that are highly effective at breaking down ozone (O₃) when exposed to UV radiation.
Consequences of Ozone Layer Depletion
The consequences of ozone depletion are profound, with the most notable being an increase in skin cancer occurrences and cataracts in humans due to enhanced UV exposure. Additionally, it negatively affects crops, marine life, and ecosystems, leading to reduced agricultural productivity and compromised food security.
In conclusion, maintaining the integrity of the ozone layer is crucial for protecting health and sustaining agricultural and marine environments, highlighting the necessity for global cooperation in combating ozone-depleting substances.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
What is the Ozone Layer?
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The ozone layer is a protective layer in the atmosphere that absorbs harmful UV rays.
Detailed Explanation
The ozone layer is a region in the Earth's stratosphere that contains a high concentration of ozone (O₃) molecules. This layer plays a crucial role in protecting life on Earth by absorbing the majority of the sun's harmful ultraviolet (UV) radiation. Without the ozone layer, these harmful rays would reach the surface, leading to increased risks of skin cancer and other negative health effects.
Examples & Analogies
Think of the ozone layer as a natural sunscreen for our planet. Just like wearing sunscreen protects your skin from getting burned by the sun, the ozone layer protects all living things from the harmful effects of UV radiation.
Causes of Ozone Depletion
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Ozone depletion is caused by CFCs (chlorofluorocarbons) from refrigerators, sprays, etc.
Detailed Explanation
Chlorofluorocarbons (CFCs) are man-made chemicals that were commonly used in refrigerators, air conditioners, and aerosol sprays. When these substances are released into the atmosphere, they eventually rise up to the stratosphere. Here, they are broken down by the sun's UV radiation, releasing chlorine atoms that deplete ozone molecules, reducing the thickness of the ozone layer.
Examples & Analogies
Imagine CFCs as tiny thieves sneaking into a bank (the ozone layer) to steal money (ozone molecules). Each time they break in, they take away some of the wealth that protects us, which leads to a weaker defense against UV rays.
Consequences of Ozone Depletion
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Consequences: Increases skin cancer, harms crops and marine life.
Detailed Explanation
The depletion of the ozone layer allows more UV radiation to reach the Earth's surface. Increased exposure to UV rays can lead to a rise in skin cancer rates among humans, as UV radiation damages the skin cells. Additionally, it can negatively impact ecosystems; crops may suffer from reduced yields due to harmful UV exposure, and marine life, especially phytoplankton, which is critical to oceanic food webs, can be adversely affected.
Examples & Analogies
Consider the effects of too much sunlight on a garden. Just like plants can wilt and suffer when they get too much direct sunlight, all plants and animals on Earth face risks when UV rays penetrate deeper due to a thinner ozone layer.
Key Concepts
-
Ozone Layer: Protects life on Earth by absorbing UV radiation.
-
CFCs: Major contributors to ozone depletion.
-
UV Rays: Cause harmful effects including skin cancer.
Examples & Applications
The ozone layer absorbs approximately 98% of the Sun's harmful ultraviolet radiation.
The Montreal Protocol, signed in 1987, is an international treaty aimed at eliminating substances that deplete the ozone layer.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Ozone up high, keeps us safe from the sky. Without it near, skin cancer is here!
Stories
Once upon a time, a group of friendly clouds teamed up with the ozone layer to shield the Earth from the sun's harsh rays, ensuring that all creatures could thrive peacefully.
Memory Tools
Remember ‘CFC’ as ‘Chlorine For Clouds!’ to recall that CFCs bring harm to the protective clouds of the ozone layer.
Acronyms
OZONE = Ozone’s Zone of No Exposure—the area that prevents harmful rays from hitting Earth.
Flash Cards
Glossary
- Ozone Layer
A region of Earth's stratosphere that absorbs most of the Sun's harmful ultraviolet (UV) radiation.
- CFCs
Chlorofluorocarbons, compounds used in air conditioning and aerosol sprays that are harmful to the ozone layer.
- UV Rays
Ultraviolet rays, a form of electromagnetic radiation that can cause skin damage and health issues.
Reference links
Supplementary resources to enhance your learning experience.
