Density
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Density
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're learning about density! Density is a measure of how much mass is contained in a certain volume. Can anyone tell me the formula for calculating density?

Is it Mass divided by Volume?

Exactly! So, the formula is Density = Mass/Volume. Remember, 'Density is Delicious!' – this helps us remember the formula: D = M/V. Now, what are the units for density?

Isn’t it kg/m³ for SI units?

Correct! And in the CGS system, it's g/cm³. Great job! Now let's move on to how we measure the density of different objects.
Measuring Density of Solids
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s discuss measuring density, starting with regular solids. Who knows how to find the volume of a cube or rectangular solid?

We can use length × width × height!

Excellent! After calculating the volume, we weigh the object for mass. Then we can apply our formula. What about an irregular solid?

We can use the water displacement method with a Eureka can!

That’s right! After measuring the volume displaced, we can proceed with finding the mass and calculating the density. Alright, let’s summarize these points. Measuring density involves mass and volume calculations.
Density of Liquids
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now we'll look into measuring the density of liquids. How do we find the volume of a liquid?

We use a measuring cylinder!

Correct! For liquids, we measure the mass of the filled container and subtract it from the empty container's mass. What units do we often use?

g/cm³!

Good! Remember, density for liquids also helps us understand whether substances will float or sink. Let’s finish up today by discussing relative density.
Relative Density and Floating and Sinking
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Relative density compares a substance's density to that of water. Why might this be useful?

It helps us predict if an object will float or not!

That's right! If the density of an object is less than water, it will float, and if it’s more, it will sink. Can someone think of an example of this?

An ice cube floats in water but a rock sinks!

Perfect example! So, to wrap up, density plays a crucial role in real life, especially when we think about objects in fluids.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section discusses the concept of density, including its definition, formula, and units of measurement, as well as the methods for measuring density for regular solids, irregular solids, and liquids. Additionally, it introduces the related concepts of relative density and the principles that govern floating and sinking.
Detailed
Density
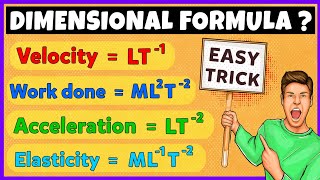
Density is a fundamental physical quantity that represents the mass of a substance divided by its volume. It is mathematically expressed by the formula Density = Mass / Volume. Density is measured in various units, with the SI unit being kg/m³ and the CGS unit being g/cm³. Understanding density is crucial for differentiating materials and predicting whether they will float or sink in a fluid.
Types of Density Measurements
Regular Solids
- For measuring the density of regular solids, one must calculate the volume based on the object's dimensions, measure its mass using a balance, and then apply the density formula.
Irregular Solids
- To find the density of irregular solids, a technique such as water displacement (using a Eureka can) is employed to measure volume. The mass is determined similarly, and the density is calculated.
Liquids
- The measurement of density for liquids involves using a measuring cylinder to determine volume, while mass is measured by comparing the weight of an empty container and a filled one.
Additional Concepts
- Relative Density: Defined as the ratio of the substance's density to the density of water, this dimensionless measure assists in understanding buoyancy.
- Floating and Sinking: An object will float if its density is less than the fluid and sink if its density is greater.
This section is significant as it lays the groundwork for further exploration of principles such as Archimedes' Principle and the Law of Floatation.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Density
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Definition: Density is mass per unit volume.
Detailed Explanation
Density is a measure that tells us how much mass is contained in a certain volume. It gives us an idea of how compact or spread out the material is. For example, if you have a block of wood and a piece of metal that are the same size, the density will help you understand why the metal feels heavier than the wood.
Examples & Analogies
Think of density like packing a suitcase. If you stuff your suitcase full of clothes (higher mass) but keep the same size (volume), it will be much heavier than if you only put a few clothes in it (lower mass). So, the weight you can carry will depend on how much you pack into the suitcase!
Formula for Density
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Formula: Density = Mass / Volume
Detailed Explanation
The formula for density helps us calculate the density of any object as long as we know its mass and volume. If you have a block that weighs 10 kilograms and takes up a volume of 2 cubic meters, you would calculate the density by dividing 10 kg by 2 m³. This would give you a density of 5 kg/m³.
Examples & Analogies
Imagine you're making smoothies. If you have 500 grams of fruit (mass) mixed into 1 liter of water (volume), you can think of density as the richness of your smoothie. The denser the mixture is, the more fruit you have in it relative to the water!
Units of Density
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Units:
○ SI Unit: kg/m³
○ CGS Unit: g/cm³
Detailed Explanation
Density can be expressed in different units depending on the system used. The International System of Units (SI) uses kilograms per cubic meter (kg/m³), while the Centimeter-Gram-Second (CGS) system uses grams per cubic centimeter (g/cm³). Understanding these units is important for converting measurements and comparing densities from different sources.
Examples & Analogies
Think of these units as different ways to measure ingredients in a recipe. Sometimes, you might use cups for measuring flour, but in a different kitchen, they might use grams. Just as it's important to know how much flour you need in either measurement, it's crucial to know density in both kg/m³ and g/cm³!
Key Concepts
-
Density: The mass of an object per unit of volume.
-
Relative Density: The comparison of a substance's density to the density of water.
-
Buoyancy: The ability of an object to float or sink in a fluid based on density.
Examples & Applications
If you have a wooden block and a metal block of the same size, the metal block will generally have a higher density and thus would sink in water.
When measuring a rock, if its mass is 200 grams and its volume is 50 cm³, its density would be 4 g/cm³.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Density is mass divided by volume, Without it in mind, your understanding is gloom!
Stories
Once there was a buoyant boat, that refused to float. Its density was too heavy, and hence it sank to the bottom!
Memory Tools
To remember Density: 'Daring Mice Venture' = Density = Mass / Volume.
Acronyms
D.M.V. = Density = Mass/Volume.
Flash Cards
Glossary
- Density
A measure of mass per unit volume of a substance.
- Mass
The quantity of matter contained in an object.
- Volume
The amount of space an object occupies.
- Relative Density
The ratio of the density of a substance to the density of water.
Reference links
Supplementary resources to enhance your learning experience.
