Properties of Bulk Matter
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Bulk Matter
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome everyone! Today, we are going to explore the properties of bulk matter, starting with what it actually is. Can anyone explain what bulk matter refers to?

Is it something related to a lot of molecules coming together?

Exactly! Bulk matter consists of materials comprised of a large number of atoms or molecules. Its properties emerge from the collective behavior of these particles. Let’s categorize these materials into solids, liquids, and gases. Who can tell me about the differences between these states?

I think solids have a fixed shape and volume because their particles are tightly packed.

Great job! And how about liquids?

They have a definite volume but take the shape of their container, right?

Correct! Finally, what about gases?

Gases have neither a fixed shape nor a volume, and the particles are pretty far apart!

Excellent understanding! Remember the acronym **S-L-G** for solid, liquid, and gas to keep them in mind. Let’s summarize: bulk matter is the collective behavior of numerous particles classified into solids, liquids, and gases.
Density and Relative Density
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss density. Who can define density for us?

Isn’t density mass per unit volume?

Right! The formula is density = mass/volume and is measured in kg/m³ or g/cm³. Can someone explain relative density?

It’s the comparison of a substance's density to the density of water?

Exactly! Relative density is a ratio and has no units. Let’s do a quick exercise. If a substance has a density of 2 g/cm³, what’s its relative density in comparison to water?

That would be 2 because the density of water is 1 g/cm³.

Perfect! Remember, relative density helps us understand whether an object will float or sink in water.
Pressure and Its Applications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s move on to pressure. Who can tell me about the formula for pressure?

Pressure is force per unit area, right? So P = F/A.

Good! And what is the SI unit for pressure?

It’s the Pascal, or Pa!

Correct! Pressure behaves in specific ways; it’s directly proportional to force and inversely proportional to area. Can anyone give an example of this principle?

Maybe how a sharp knife cuts better? A smaller area results in higher pressure?

Exactly! And how about broad tires for soft surfaces?

They have a larger area, giving lower pressure to avoid sinking!

Well explained! So, pressure is crucial in many applications. Let’s recap—we learned pressure is force per unit area, with significant implications in design.
Buoyancy and Archimedes' Principle
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, we have buoyancy and Archimedes’ Principle. Can anyone describe what buoyancy is?

It’s the upward force on an object immersed in a fluid!

Correct! And what does Archimedes’ Principle state?

It says that the upward force equals the weight of the displaced fluid.

Exactly! This principle explains why some objects float while others sink. Can you think of an example where this applies?

Designing ships and submarines!

Right! Buoyancy is crucial for various applications in engineering. Let’s summarize that buoyancy is the upward force experienced in fluids, based on how much fluid is displaced.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, we dive into the properties of bulk matter, discussing solid, liquid, and gas states, their densities, pressure exertion, buoyancy, surface tension, viscosity, capillarity, elasticity, and practical applications. Understanding these properties helps explain everyday phenomena and their real-world applications.
Detailed
Properties of Bulk Matter
In this chapter, we delve into the fascinating world of bulk matter, which encompasses materials composed of a large number of atoms or molecules. The behavior of particles collectively gives rise to the properties that distinguish solids, liquids, and gases.
States of Matter
We categorize matter into three primary states:
1. Solid: Defined shape and volume, with tightly packed particles.
2. Liquid: Fixed volume but no set shape, allowing particles to flow loosely.
3. Gas: Neither fixed shape nor volume, with particles moving freely apart.
Density and Relative Density
Density is defined as mass per unit volume (D = M/V), expressed in kg/m³ or g/cm³. Relative density refers to the density ratio of a substance to water, a dimensionless quantity.
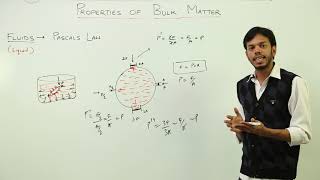
Pressure and Fluids
Pressure is the force exerted per unit area (P = F/A), measured in Pascals (Pa). It behaves according to fundamental principles, being inversely proportional to area and directly proportional to force. Fluids exert pressure in all directions and exhibit hydrostatic pressure that increases with depth, calculated as P = hρg (h = depth, ρ = density, g = gravity).
Buoyancy and Archimedes’ Principle
Buoyancy is the upward force on an immersed object, determined by Archimedes’ Principle, which states that it equals the weight of the fluid displaced. Applications include ship design and submarines.
Surface Tension
Surface tension describes the elastic-like force at the surface of a liquid, primarily due to cohesive forces among molecules. This explains phenomena such as insects walking on water and droplet formation.
Viscosity and Capillarity
Viscosity indicates a liquid's resistance to flow, affected by temperature and the liquid's nature. Capillarity pertains to a liquid's movement in narrow spaces, seen in ink pens and plant roots.
Elasticity
Elasticity is the ability of a material to return to its original shape after deformation, described through stress and strain concepts. Young’s Modulus relates stress to strain, with rubber exemplifying elasticity versus clay, which shows plasticity.
Applications of Bulk Properties
Understanding these properties aids in designing hydraulic systems, capillary actions relevant in nature and technology, and leveraging surface tension in liquids such as detergents and paints.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Bulk Matter
Chapter 1 of 11
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Bulk matter refers to materials composed of a large number of atoms or molecules.
● The properties of such materials arise from the behavior of particles as a whole rather than individually.
● This chapter focuses on solids, liquids, and gases.
Detailed Explanation
Bulk matter consists of materials that are made up of a large number of atoms or molecules. Unlike individual atoms or small groups of molecules whose properties can be very different, bulk matter behaves in a way that reflects the collective behavior of all the particles contained within it. For example, if we consider water, the properties such as boiling, freezing, and its ability to dissolve substances are a result of the interactions between millions of water molecules, rather than just one water molecule's characteristics. In this chapter, we will explore the properties of three states of bulk matter: solids, liquids, and gases, examining how these properties differ due to the arrangement and interaction of their particles.
Examples & Analogies
Think of bulk matter like a group of friends at a concert. Each individual might behave differently when alone, but together, as a big group, they create a unique atmosphere. Just like how the concert experience is a result of all the friends coming together, the properties of bulk matter emerge from the interactions of large numbers of particles.
States of Matter
Chapter 2 of 11
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Solid: Fixed shape and volume; particles are tightly packed.
● Liquid: Fixed volume, no fixed shape; particles loosely packed and can flow.
● Gas: No fixed shape or volume; particles are far apart and move freely.
Detailed Explanation
Bulk matter exists in three primary states: solids, liquids, and gases, each distinguished by the arrangement and behavior of their particles. In solids, particles are closely packed together in a fixed arrangement, giving solids a definite shape and volume. For example, a cube of ice retains its shape. Liquids have a fixed volume but no fixed shape, meaning they flow and take the shape of their container. Water is a great example; it stays a certain amount in a cup but takes the shape of the cup. Gases have neither fixed shape nor volume; their particles are far apart and move freely, expanding to fill any container, such as the smoke from a fire spreading in the air.
Examples & Analogies
Imagine you have three boxes: one filled with marbles (solid), one filled with water (liquid), and one filled with air (gas). The marbles in the first box stay in a specific arrangement and shape, while the water fills the bottom of the box but takes the shape of the box's sides. The air in the third box moves around freely, filling every corner of the box. This illustrates the differences in the states of bulk matter!
Density and Relative Density
Chapter 3 of 11
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Density: Mass per unit volume.
○ Formula: Density = Mass / Volume
○ SI Unit: kg/m³ (or g/cm³)
● Relative Density:
○ Ratio of the density of a substance to the density of water.
○ Formula: Relative Density = Density of substance / Density of water
○ It has no unit.
Detailed Explanation
Density is a key property of bulk matter and describes how much mass is contained in a given volume. The formula for density is simple: you divide the mass of an object by its volume. For example, if you have a block of wood that weighs 2 kg and occupies 1 m³ of space, its density would be 2 kg/m³. Relative density compares the density of a substance to the density of water, indicating whether it will float or sink in water. Since water has a density of 1 g/cm³, if a substance has a relative density less than 1, it will float, while one greater than 1 will sink.
Examples & Analogies
Think of density like a sponge and a rock. If you have a sponge and a rock of the same size, the rock is much heavier, meaning it's denser. The sponge is less dense and floats on water, just like substances with a relative density less than water. A simple test is when you put both in water; the sponge floats while the rock sinks. This is the practical effect of density and relative density!
Pressure
Chapter 4 of 11
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Pressure: Force applied per unit area.
○ Formula: Pressure = Force / Area
○ SI Unit: Pascal (Pa)
● Factors affecting pressure:
○ Inversely proportional to area.
○ Directly proportional to force.
● Applications:
○ Sharp knife cuts better (small area, more pressure).
○ Broad tires for soft surfaces (large area, less pressure).
Detailed Explanation
Pressure is defined as the force applied to a surface divided by the area of that surface. This means if you apply the same force over a smaller area, you'll have higher pressure; if it's applied over a larger area, the pressure is reduced. For example, a sharp knife have a small blade area, allowing it to exert a lot of pressure on the surface it's cutting, making it effective at slicing. Conversely, broad tires on vehicles are designed to exert less pressure on soft ground, preventing them from sinking.
Examples & Analogies
Imagine standing on a soft beach with and without shoes. If you walk barefoot, your feet exert a larger area of pressure and you sink slightly into the sand. But if you wear a pair of shoes, which has a smaller surface area compared to your feet, they exert more pressure per square inch and you sink less. This illustrates how pressure varies with area!
Pressure in Fluids
Chapter 5 of 11
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Fluids (liquids and gases) exert pressure in all directions.
● Hydrostatic Pressure: Pressure exerted by a fluid at rest.
○ Increases with depth.
○ Formula: P = hρg
■ h = depth, ρ = density, g = gravity
● Atmospheric Pressure:
○ Pressure exerted by air above the Earth.
○ Standard atmospheric pressure = 1 atm = 1.013 × 10⁵ Pa.
Detailed Explanation
Fluids, which include both liquids and gases, exert pressure in every direction. For example, when you dive underwater, the deeper you go, the more pressure you feel due to the weight of the water above you, which is known as hydrostatic pressure. The formula P = hρg shows how this pressure increases with depth (h), the density of the fluid (ρ), and the acceleration due to gravity (g). Additionally, atmospheric pressure is the weight of the air above us, and its standard value at sea level is 1 atm or 101,325 Pa.
Examples & Analogies
Think of being at the bottom of a pool. The deeper you go, the heavier the water above presses down on your body, just like the weight of the atmosphere presses down on us all the time. You can imagine the atmosphere is like a big blanket made of air that covers the Earth, and the deeper you go into the water, the more the blanket of water presses down on you!
Buoyancy and Archimedes’ Principle
Chapter 6 of 11
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Buoyancy: The upward force experienced by an object immersed in a fluid.
● Archimedes’ Principle:
○ A body immersed in a fluid experiences an upward force equal to the weight of the fluid displaced.
● Applications:
○ Designing ships, submarines.
○ Determining relative density.
Detailed Explanation
Buoyancy is the upward force that enables objects to float in a fluid. Archimedes' Principle states that when a body is submerged in a fluid, it displaces a volume of fluid equivalent to its own weight, resulting in this buoyant force. That's why boats can float; they displace an amount of water that weighs as much as the boat itself. This principle is also used in designing submarines, which can control their buoyancy to float or sink as needed.
Examples & Analogies
Imagine getting into a swimming pool. When you jump in, you displace some water, and you feel the force pushing you back up to the surface — that’s buoyancy at work! If you get a beach ball and try to push it under the water, you'll notice it keeps wanting to pop back up. This is because it displaces water equal to its weight, demonstrating Archimedes' Principle in action!
Surface Tension
Chapter 7 of 11
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Tendency of the liquid surface to behave like a stretched elastic sheet.
● Caused by cohesive forces between molecules.
● Explains:
○ Why insects walk on water.
○ Formation of droplets.
Detailed Explanation
Surface tension is the property that makes the surface of a liquid behave like a stretched elastic sheet. This is due to the cohesive forces between the molecules within the liquid, pulling them together. For example, water molecules stick tightly together at the surface, creating a 'film' that allows small insects, like water striders, to walk on water without sinking. Surface tension also causes water to form droplets on surfaces instead of spreading out.
Examples & Analogies
Think of a soap bubble or the way raindrops bead up on a leaf. The cohesive forces among the liquid molecules pull them together, making them take shape. Just like how a tightrope walker balances on a thin wire, water’s surface tension allows small objects to 'walk' on it, demonstrating the unique properties of liquids!
Viscosity
Chapter 8 of 11
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Resistance to the flow of a liquid.
● More viscosity → slower flow (e.g., honey).
● Less viscosity → faster flow (e.g., water).
● Depends on:
○ Temperature (inversely related).
○ Nature of liquid.
Detailed Explanation
Viscosity measures a liquid's resistance to flow. A liquid with high viscosity, like honey, flows very slowly due to its thick consistency, while low-viscosity liquids, like water, flow much more easily. Viscosity can change with temperature; generally, heating a liquid decreases its viscosity, allowing it to flow more freely. This principle explains why syrup flows better when warmed up compared to when it's cold.
Examples & Analogies
Think of pouring different liquids. When you pour honey, it takes a while to come out—the thickness makes it hard to flow. But when you pour water, it comes out quickly. It’s like trying to run in mud versus running on a smooth surface; the mud (high viscosity) makes it difficult to move, just like honey does!
Capillarity
Chapter 9 of 11
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Rise or fall of a liquid in a narrow tube due to adhesive and cohesive forces.
● Water rises in a capillary tube; mercury falls.
● Used in:
○ Ink pens, plant roots absorbing water.
Detailed Explanation
Capillarity is the movement of a liquid in a narrow space, such as when water rises in a thin tube. This phenomenon occurs due to adhesive forces, which attract the liquid to the surfaces of the tube, and cohesive forces, which keep the liquid molecules together. Water will rise in a capillary tube due to these forces, while mercury behaves differently and falls due to its strong cohesive forces. Capillarity is also important for plants, allowing them to draw water up from their roots.
Examples & Analogies
Imagine a straw in a glass of water. When you suck on the straw, the water rises up into it, which is a result of capillary action. Just like how a sponge soaks up water from a puddle, plants use this same principle to pull water from the soil up through their roots to their leaves!
Elasticity
Chapter 10 of 11
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Property of a body to regain its original shape after removing deforming force.
● Stress: Force per unit area.
● Strain: Deformation per unit length.
● Young’s Modulus: Ratio of stress to strain.
● Rubber is elastic; clay is plastic (no elasticity).
Detailed Explanation
Elasticity is the ability of materials to return to their original shape after being deformed by a force. When you stretch a rubber band and release it, it snaps back to its original shape—this is elasticity in action. Stress refers to the force applied to the material, while strain describes how much it deforms. Young’s Modulus quantifies the relationship between stress and strain for materials. Elastic materials, like rubber, can stretch and return, while plastic materials, like clay, cannot return to their original shape once deformed.
Examples & Analogies
Think of a spring. If you stretch it and let go, it bounces back to its original shape—this represents elasticity. In contrast, if you squish a piece of clay into a different shape, it stays that way, showing how some materials can’t return to their original form like rubber can. This helps us understand why certain materials are used for specific applications based on their elastic properties!
Applications of Bulk Properties
Chapter 11 of 11
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Designing hydraulic lifts and brakes.
● Capillary action in ink pens and plants.
● Surface tension in detergents and paints.
● Elasticity in construction and sports gear.
Detailed Explanation
The properties of bulk matter have numerous applications in everyday life and technology. For example, hydraulic lifts utilize the principles of pressure and fluid mechanics to lift heavy loads efficiently. Capillary action is important for ink pens, allowing ink to flow to the tip, and for plants, aiding in water absorption from the soil. Surface tension is exploited in soaps and detergents to enhance their effectiveness. Elasticity is crucial in construction materials and sports equipment, providing the right balance of strength and flexibility.
Examples & Analogies
Consider a hydraulic lift, like those found in car repair shops. They lift heavy cars by applying pressure to fluid in a closed system, demonstrating how pressure allows for efficient lifting. Similarly, when you use a fountain pen, the ink moves due to capillary action, just like how a sponge absorbs water. These examples show how the properties of bulk matter are not just theoretical but are actively used in technology and nature!
Key Concepts
-
States of Matter: Classification of matter into solids, liquids, and gases based on their properties.
-
Density: Pertains to mass per unit volume, crucial for understanding buoyancy.
-
Pressure: Force per unit area, significant in various applications like hydraulics.
-
Buoyancy: Upward force experienced by submerged objects, essential for fluid dynamics.
-
Archimedes' Principle: Indicates the weight of fluid displaced determines buoyancy.
-
Viscosity: Resistance to flow, determining how liquids behave under stress.
-
Surface Tension: The cohesive force at a liquid's surface, influencing many natural phenomena.
-
Elasticity: The ability of an object to return to its original shape after deformation.
Examples & Applications
Ice floating on water demonstrates buoyancy since its relative density is less than water.
Surface tension allows water striders to walk on the surface of ponds without sinking.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
If it's solid, it won't change, liquids flow, gases rearrange.
Stories
Imagine a fish in the water, it floats and dances with ease, thanks to buoyancy lifting it up like a breeze!
Memory Tools
D.B.P.S.V.C.E - Density, Buoyancy, Pressure, Surface tension, Viscosity, Capillarity, Elasticity - The properties we see!
Acronyms
SLG - Solid, Liquid, Gas – the three states of matter.
Flash Cards
Glossary
- Bulk Matter
Materials composed of a large number of atoms or molecules.
- Density
Mass per unit volume of a substance.
- Relative Density
Ratio of the density of a substance to the density of water.
- Pressure
Force applied per unit area.
- Buoyancy
Upward force experienced by an object immersed in a fluid.
- Archimedes’ Principle
A body immersed in a fluid experiences an upward force equal to the weight of the fluid displaced.
- Surface Tension
The elastic-like force at the surface of a liquid.
- Viscosity
Resistance of a liquid to flow.
- Capillarity
Movement of a liquid in narrow spaces due to adhesive and cohesive forces.
- Elasticity
Ability of a material to return to its original shape after deformation.
Reference links
Supplementary resources to enhance your learning experience.
