Atomic Size (Radius)
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Atomic Size
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we will explore atomic size, specifically what it is and how it changes across the periodic table. Can anyone define what we mean by atomic size?

I think it's how big an atom is, but how do we measure it?

Great question! It's measured as atomic radius, which is the distance from the nucleus to the outermost electrons. Now, why do you think understanding atomic size is important?

I guess it helps us know how elements will behave when they react?

Exactly! Knowing atomic size helps predict chemical reactivity and interaction. Let's dive into the specific trends.

What are those trends?

There are two main trends—decreasing size across a period and increasing size down a group. Let’s discuss these!
Size Decrease Across a Period
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

First, as you move from left to right in a period, the atomic size decreases. Who can tell me why that happens?

Is it because there are more protons pulling the electrons in?

Exactly! Increased nuclear charge pulls the electron cloud closer, which decreases the radius. Remember the phrase ‘Pulling In’ to help you recall this trend.

What about helium versus sodium?

That's a great observation! Helium, with a higher positive charge from its nucleus, pulls its electrons in more tightly compared to sodium, resulting in a smaller atomic radius. Now, what happens to the size when we move down a group?
Size Increase Down a Group
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

As we move down a group, the atomic size increases. Can anyone explain why this occurs?

Is it because there are more layers of electrons?

Correct! Each time you go down a group, you add a new electron shell, which means electrons are further from the nucleus. Picture a tree growing taller with each new layer of soil. What might this mean for the chemical properties of elements down the group?

They might be more reactive since they can lose outer electrons more easily?

Exactly! Larger atoms tend to lose electrons more easily due to their distance from the nucleus. This aspect really influences reactivity!
Recap and Summary
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

So, let's summarize what we learned about atomic size. Who can tell me about the trend across a period?

Atomic size decreases from left to right because of increased nuclear charge pulling electrons closer.

And what happens down a group?

It increases because we add more electron shells.

Excellent! Remember these trends as they are crucial for understanding the reactivity of different elements.

Thanks, this really clears things up!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
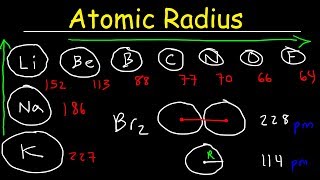
Atomic size, or atomic radius, refers to the distance from the center of the nucleus to the outermost electrons. It decreases as you move from left to right across a period due to increased nuclear charge and increases as you move down a group due to additional electron shells.
Detailed
Atomic Size (Radius) in the Modern Periodic Table
Atomic size, measured as atomic radius, varies systematically within the periodic table. This section outlines two primary trends:
- Decreasing Atomic Size Across a Period: As you move left to right in a period, the atomic number increases, leading to a greater positive charge in the nucleus. This increased nuclear charge pulls the electron cloud closer to the nucleus, thus reducing the atomic radius.
- Memory Aid: Remember the phrase “Pulling In” to recall that increased nuclear charge pulls electrons closer, decreasing size.
- Increasing Atomic Size Down a Group: When moving down a group, each consecutive element has an additional electron shell, which outweighs the increase in nuclear charge. The increased distance from the nucleus means that the outermost electrons are further away, resulting in a larger atomic radius.
- Memory Aid: Picture a tree growing taller as you add more soil layers; more shells lead to a bigger atom.
Understanding atomic size is crucial in predicting how elements will react chemically and interact with one another.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Trend of Atomic Size Across a Period
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
• Decreases across a period (left to right)
Detailed Explanation
As you move from left to right across a period in the periodic table, the atomic size of the elements decreases. This happens because the number of protons in the nucleus increases, resulting in a greater positive charge. This stronger positive charge attracts the electrons more closely towards the nucleus, pulling the electron cloud in and reducing the size of the atom.
Examples & Analogies
Think of it like a magnet attracting smaller metal objects. As you bring a stronger magnet (more protons) closer to small metallic pieces (electrons), the pieces get pulled in closer, demonstrating how increased attraction reduces the overall size.
Trend of Atomic Size Down a Group
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
• Increases down a group
Detailed Explanation
As you move down a group in the periodic table, the atomic size of the elements increases. This is because with each subsequent element down a group, a new electron shell is added. Each shell is further from the nucleus, meaning the overall distance from the nucleus to the outermost electron increases, resulting in a larger atomic size.
Examples & Analogies
Imagine a multi-story building where each floor represents a new electron shell. As you go up each floor (moving down a group), you are getting further away from the ground floor (nucleus). The higher you go, the larger the building becomes, just as the atomic size increases down a group.
Key Concepts
-
Atomic Radius: The distance from the nucleus to the outermost electrons.
-
Trend Across a Period: Atomic radius decreases from left to right due to increased nuclear charge.
-
Trend Down a Group: Atomic radius increases down a group due to the addition of electron shells.
Examples & Applications
The atomic radius of lithium (Li) is larger than that of fluorine (F) because fluorine has such a high nuclear charge that it pulls its electrons closer.
When comparing sodium (Na) and potassium (K), potassium has a larger atomic radius due to additional electron shells.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Across a period, small is the trend, nuclear charge its pull does send.
Stories
Imagine climbing a mountain; as you go higher, you feel the air pulling you closer, much like nuclear charge pulls electrons in across a period.
Memory Tools
PULLS IN for the decrease across a period and MORE SHELLS for the increase down a group.
Acronyms
RAMP
Radius And Movement Periodically – Remember how atomic size changes regarding periods and groups.
Flash Cards
Glossary
- Atomic Radius
The distance from the center of the nucleus to the outermost electrons.
- Period
Horizontal rows in the periodic table.
- Group
Vertical columns in the periodic table.
- Nuclear Charge
The total charge of the nucleus, determined by the number of protons.
Reference links
Supplementary resources to enhance your learning experience.
