Metallic and Non-metallic Character
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Metallic Character
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to explore metallic character. Can anyone tell me what metallic character means?

Is it about how elements can lose electrons or something?

Exactly, Student_1! Metallic character refers to the tendency of an element to lose electrons and form positive ions. Now, can anyone share how metallic character changes from left to right across the periodic table?

It decreases because atoms become more non-metallic as you move to the right?

Correct! As we move across a period, elements become less metallic because they tend to gain electrons instead. Let's remember this with the mnemonic: 'Left Loses, Right Gains' to signify that elements on the left are more metallic.

What about going down a group?

Good question! Moving down a group, metallic character actually increases because the outer electrons are further from the nucleus, making them easier to lose. This is how we understand the periodic trends!

So heavier elements are more metallic?

Correct, Student_4! Heavier elements, located lower in a group, display stronger metallic character due to lower ionization energy.

To summarize, metallic character decreases across a period and increases down a group. Remember: 'Left Loses' for the trend across periods and 'Down Goes' for the trend down groups!
Exploring Non-Metallic Character
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's shift focus to non-metallic character. Who can define it for us?

I think it's about elements gaining electrons, right?

That's right, Student_1! Non-metallic character signifies the ability of an element to gain electrons and form negative ions. How does this character vary as we go across a period?

Isn't it the opposite of metallic character? So it should increase as we move right?

Exactly! Non-metallic character does increase across a period. Remember, 'Right Gains, Left Loses' to help you recall these trends. Now, what happens down a group?

It decreases, right?

Correct! Non-metallic character decreases down a group as elements become more metallic. Remember, as you go down, it’s easier to lose electrons as the effective nuclear charge weakens. Can anyone summarize what we've discussed about non-metallic character?

Non-metallic character increases across a period and decreases down a group!

Great recap! In summary, non-metallic character increases from left to right across the periodic table and decreases from top to bottom.
Comparative Trends in Metallic and Non-metallic Character
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s review the trends of both characters we discussed. Why is it important to understand the relationship between these two?

Understanding them helps us predict how elements will react, right?

Exactly! The metallic and non-metallic characters influence chemical reactivity. For instance, metals are more likely to react with non-metals. Can someone remind us of the trends we've learned?

Metallic character decreases across a period and increases down a group.

And non-metallic character does the opposite!

Correct! 'Metal to Non-metal, Left to Right' indicates the switch from metallic to non-metallic. Therefore, understanding these characters is crucial for understanding element interactions!

So, knowing these trends also helps in predicting bonding types?

Absolutely! Elements combine based on their metallic and non-metallic characters, influencing ionic and covalent bond formations. Great job everyone!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
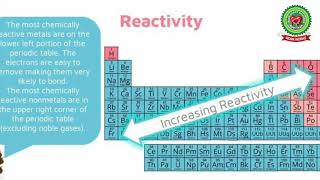
The section explains how metallic character decreases across a period and increases down a group, while non-metallic character shows the opposite trend, increasing across a period and decreasing down a group. Understanding these trends helps in predicting the behavior of elements.
Detailed
In this section, we explore the differences in metallic and non-metallic character within the periodic table. Metallic character refers to the tendency of an element to lose electrons and form positive ions or cations, while non-metallic character relates to the ability to gain electrons and form negative ions or anions. As we move from left to right across a period, the metallic character decreases because the elements are more inclined to gain electrons rather than lose them, leading to a higher non-metallic character. Conversely, as we move down a group, the metallic character increases because additional electron shells make it easier for atoms to lose electrons due to lower effective nuclear charge. This understanding of periodic trends is crucial for predicting the properties and reactions of elements.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Overview of Metallic and Non-metallic Character
Chapter 1 of 1
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Metallic character decreases across a period
- Increases down a group
- Non-metallic character increases across a period, decreases down a group
Detailed Explanation
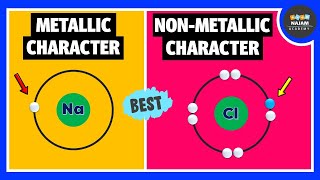
In this section, we will discuss the trends in metallic and non-metallic character of elements in the periodic table. As we move from left to right across a period, the metallic character of elements decreases. This means that elements on the left side of the periodic table are more metallic in nature compared to those on the right. For example, sodium (Na) is more metallic than chlorine (Cl), which is found on the right side of the periodic table. Conversely, as we move down a group from top to bottom, the metallic character increases. This means that elements at the bottom of a group, like cesium (Cs), are more metallic than those at the top, like lithium (Li).
On the other hand, non-metallic character shows an opposite trend. Non-metallic character increases as we move from left to right across a period, meaning that elements such as oxygen (O) are more non-metallic than sodium (Na). However, as we move down a group, the non-metallic character decreases, indicating that elements lower down, like iodine (I), are less non-metallic than those higher up, like fluorine (F).
Examples & Analogies
Think of metallic character like a team of players. The players on the left side of the field (the left side of the periodic table) are very aggressive and competitive (more metallic), while those on the right side are more reserved and strategic (less metallic). When you look at players on the same team who are further back in the formation, they tend to be bigger and more aggressive (more metallic) compared to the front players, especially if you compare them to the smaller, second-string players at the front (non-metallic). In contrast, think of how certain materials react; metals like sodium are very reactive like aggressive players, while non-metals like oxygen are less reactive and more strategic in their interactions.
Key Concepts
-
Metallic Character: Tendency to lose electrons and form positive ions.
-
Non-metallic Character: Ability to gain electrons and form negative ions.
-
Trend Across Periods: Metallic character decreases, non-metallic character increases.
-
Trend Down Groups: Metallic character increases, non-metallic character decreases.
Examples & Applications
Sodium (Na) has a high metallic character as it easily loses one electron to form Na+.
Chlorine (Cl) exhibits a high non-metallic character as it readily gains an electron to form Cl-.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Right is bright where non-metals gain, Left is tough, metals lose in their reign.
Stories
Imagine a battle in a kingdom. On the left, brave knights (metals) swiftly surrender their swords (electrons) to defend their castle, while gentle scholars (non-metals) on the right eagerly take the swords, ready to learn and grow strong.
Memory Tools
Remember: 'M-N' stands for Metallic decreases, Non-metallic increases across periods.
Acronyms
'MISC' - Metallic decreases, Increases down, Sulfur and others Gain.
Flash Cards
Glossary
- Metallic Character
The tendency of an element to lose electrons and form positive ions.
- Nonmetallic Character
The ability of an element to gain electrons and form negative ions.
Reference links
Supplementary resources to enhance your learning experience.
