Fuel Cells: Overview, Classification, Operating Principles, and Thermodynamics
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Overview of Fuel Cells
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, let's discuss what fuel cells are. Fundamentally, they convert chemical energy directly into electricity. Can anyone tell me what fuels are commonly used?

Isn't hydrogen the main fuel used?

Correct! Hydrogen is indeed the most common fuel. Fuel cells can also use hydrocarbons and alcohols. Now, what do you think is the main advantage of fuel cells over batteries?

Maybe that they don’t run out of energy like batteries do?

Exactly! As long as you supply fuel and oxidant, fuel cells can continue producing electricity. So, they require continuous inputs. What do we know about their byproducts?

Water is the main byproduct, right?

That's right! This leads to low emissions, especially compared to combustion engines.

Could they really be used in cars and space?

Yes! Fuel cells are used in both cars and space applications. They’re versatile because of their scalability.

In summary, fuel cells can convert chemical energy to electricity efficiently and cleanly, which opens up many applications.
Classification of Fuel Cells
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s move on to how we classify fuel cells. What do you think determines the type of fuel cell?

Isn’t it the electrolyte used?

Precisely! The electrolyte affects the operating temperature and applications. For instance, can anyone name an example of a low-temperature fuel cell?

The Proton Exchange Membrane Fuel Cell!

Yes! PEMFCs operate between 20-100 °C. What are some of their applications?

They’re used in vehicles!

Correct. What about a higher temperature fuel cell?

The Solid Oxide Fuel Cell?

Exactly! SOFCs also allow for flexible fuel use due to their higher operating temperatures. In summary, the classification helps us understand their usability in various fields.
Operating Principles of Fuel Cells
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s delve into how fuel cells operate. Who remembers the components of a fuel cell?

There’s the anode, cathode, and electrolyte!

Correct! The anode is where the fuel is supplied. What happens to the fuel at the anode?

It gets oxidized and loses electrons!

Exactly. The electrons then flow through an external circuit, which generates electricity. What about the cathode?

It’s where the oxidizer is supplied and gets reduced.

Correct! The overall reaction leads to water as a byproduct. Who can remember the typical reaction at the anode?

H₂ goes to 2H⁺ and 2e⁻.

Well done! Each detail contributes to the overall efficiency of fuel cells, allowing them to operate effectively.
Thermodynamics of Fuel Cells
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s explore the thermodynamics involved. What do we mean by efficiency in fuel cells?

It’s the ratio of usable energy output to input energy.

Exactly! And what thermodynamic principle helps us to understand this efficiency?

The Gibbs free energy!

Right, Gibbs free energy allows us to calculate the maximum usable work from the fuel cell. Can anyone tell me the formula for cell potential?

E = -ΔG/nF!

Great! Understanding these principles is crucial in evaluating fuel cells’ performances and efficiency. Finally, about efficiency: what’s the maximum efficiency for hydrogen fuel cells?

Around 83% at standard conditions.

Exactly! However, practicality often yields efficiencies between 40-60%. You all did wonderfully today!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Fuel cells are electrochemical devices that convert chemical energy directly into electric energy, offering high efficiency and low emissions. This section explores various types of fuel cells based on their electrolytes, the principles underlying their operation, and the thermodynamic laws that govern their efficiency.
Detailed
Fuel Cells: Overview, Classification, Operating Principles, and Thermodynamics
Fuel cells are electrochemical devices that convert chemical energy from fuel, primarily hydrogen, and an oxidant (commonly oxygen) into electricity and heat through a series of redox reactions. Unlike traditional batteries, fuel cells do not store energy but require a continuous supply of reactants. This section breaks down the classification of fuel cells based on their electrolyte types, operating principles including their structure and function, and the thermodynamic principles that dictate their efficiency.
Key Features of Fuel Cells
- High Efficiency: Especially at partial loads, often achieving efficiencies between 40%-60% in practical applications.
- Low Emissions: Hydrogen fuel cells mainly emit water as a byproduct.
- Silent Operation: They have few or no moving parts, leading to quieter functioning.
- Scalability: Fuel cells can be designed to suit applications ranging from small devices to large station-based power generators.
Classification of Fuel Cells
Fuel cells are primarily classified based on the type of electrolyte they use, and this classification affects their operating temperature, fuel type, and applications. The main types include:
- Proton Exchange Membrane Fuel Cell (PEMFC): Operates at low temperatures (20-100 °C), applications in portable electronics and vehicles.
- Aqueous Alkaline Fuel Cell (AFC): Sensitive to CO₂; suitable for space applications.
- Phosphoric Acid Fuel Cell (PAFC): Used in stationary power generation (150-220 °C).
- Molten Carbonate Fuel Cell (MCFC): Higher temperature (600-700 °C) for large stationary power generation.
- Solid Oxide Fuel Cell (SOFC): Flexible fuel usage at high temperatures (500-1000 °C).
- Direct Methanol Fuel Cell (DMFC): Common in portable devices (20-120 °C).
Operating Principles
Each fuel cell features:
- Anode: Where fuel is oxidized.
- Cathode: Where the oxidant is reduced.
- Electrolyte: Conducts ions but blocks electrons, generating a usable electric current.
- Catalyst: Sometimes used to facilitate reactions effectively.
Typical Reactions:
- At the anode: (H₂ → 2H⁺ + 2e⁻)
- At the cathode: (O₂ + 4H⁺ + 4e⁻ → 2H₂O)
Thermodynamics of Fuel Cells
Fuel cell efficiency is also affected by thermodynamics, particularly Gibbs free energy. The cell's efficiency compares the usable work against the enthalpy change, with different processes having distinct theoretical and practical efficiencies.
In summary, fuel cells represent a crucial technology for clean energy conversion, directly transforming chemical energy into usable electric power with minimal environmental impact.
Youtube Videos



Audio Book
Dive deep into the subject with an immersive audiobook experience.
Overview of Fuel Cells
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A fuel cell is an electrochemical device that converts the chemical energy of a fuel (commonly hydrogen, but also including hydrocarbons, alcohols, and ammonia) and an oxidant (usually oxygen from the air) directly into electricity, heat, and water through a pair of redox reactions. Unlike batteries, which store a fixed amount of energy, fuel cells require a continuous supply of fuel and oxidant to produce electricity and can operate as long as these are supplied. Fuel cells are used in applications ranging from portable electronics and vehicles to distributed power generation, backup power, and spaceflight systems.
Detailed Explanation
A fuel cell functions by taking in fuel (like hydrogen) and an oxidant (commonly oxygen) and converting these into electricity. This happens through an electrochemical reaction, which is different from combustion methods that produce heat via burning. Unlike batteries that hold a set amount of energy until depleted, fuel cells keep generating electricity as long as fuel and oxidant are continuously supplied. This trait makes them ideal for varied applications, where a reliable power source is needed, including in vehicles, electronics, and in powering systems for space travel.
Examples & Analogies
Think of a fuel cell like a water faucet; as long as water (fuel) and electricity (oxidant) are flowing into it, you can continuously get water (electricity) out. In contrast, a battery is like a water bottle; once the water is empties, you have to refill it to get more water.
Key Benefits of Fuel Cells
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
High efficiency (especially at partial loads)
Low pollutant emissions (water is the main byproduct for hydrogen fuel)
Silent operation (few or no moving parts)
Scalability from milliwatts to megawatts.
Detailed Explanation
Fuel cells provide several advantages: they are highly efficient, especially when operating below their maximum load. The efficiency is significant because it means more usable energy is produced from the same amount of fuel. Moreover, the emissions are minimal; for hydrogen fuel cells, the primary byproduct is water, resulting in lower environmental pollution. Fuel cells operate quietly since they have few or no moving mechanical parts, making them suitable for applications where noise is a concern. Finally, their ability to scale means they can be used in devices that require very little power (like mobile gadgets) to very large systems (like a power plant).
Examples & Analogies
Imagine a kitchen appliance that runs quietly while using less energy and produces only water as a waste product—this is similar to how fuel cells function. For instance, a fuel cell can power your smartphone without the noise and pollution associated with traditional power generators.
Classification of Fuel Cells
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Fuel cells are most commonly classified according to their electrolyte type, which determines their operating temperature, power range, fuel, and application domain. The main types include:
| Type | Abbreviation | Electrolyte | Main Features & Applications | Operating Temp. (°C) |
|---|---|---|---|---|
| Proton Exchange Membrane Fuel Cell | PEMFC | Solid polymer membrane | Fast start-up, portable electronics, vehicles | 20 - 100 |
| Alkaline Fuel Cell | AFC | KOH | Space applications, sensitive to CO₂ | 20 - 80 |
| Phosphoric Acid Fuel Cell | PAFC | Phosphoric acid | Stationary and commercial combined heat/power | 150 - 220 |
| Molten Carbonate Fuel Cell | MCFC | Molten carbonate salts | Large stationary power generation | 600 - 700 |
| Solid Oxide Fuel Cell | SOFC | Solid ceramic (zirconia) | High-temp CHP, industrial, flexible fuel use | 500 - 1,000 |
| Direct Methanol Fuel Cell | DMFC | Polymer membrane (ionomer) | Portable/mobile devices, military | 20 - 120 |
| Regenerative, Microbial, and Other | Varies | Varies | Research/specialized applications | Varies |
Detailed Explanation
Fuel cells can be categorized based on the type of electrolyte they use, which impacts their operation and suitability for various applications. For instance, Proton Exchange Membrane Fuel Cells (PEMFC) use a solid polymer membrane and are efficient for portable devices, while Molten Carbonate Fuel Cells (MCFC) operate at very high temperatures and are suitable for large-scale power generation. Each type has unique operating characteristics and uses, covering a range from mobile applications to stationary power systems.
Examples & Analogies
Choosing the right fuel cell for an application is like picking the right tool for a job. For example, a PEMFC is like a compact screwdriver—perfect for tight spaces like electronic devices. In contrast, a MCFC is like an industrial power drill—better suited for heavy-duty usage in power plants.
Operating Principles of Fuel Cells
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
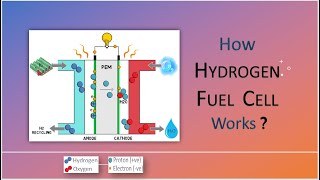
Each fuel cell consists of:
- Anode: Where fuel is supplied and oxidized, releasing electrons and ions.
- Cathode: Where oxidant is supplied and reduced, accepting electrons.
- Electrolyte: Conducts ions but blocks electrons, forcing electrons through an external circuit (providing usable electric current).
- Catalyst (optional): Often required to facilitate reactions efficiently (e.g., platinum in PEMFCs).
Typical Reaction Paths (for H₂–O₂ Fuel Cell):
- At the anode:
$ \mathrm{H_2 \rightarrow 2\mathrm{H^+ + 2\mathrm{e}^- } $
- Electrolyte:
Conducts H⁺ or OH⁻ ions from anode to cathode.
- At the cathode:
$ \mathrm{O_2 + 4\mathrm{H^+ + 4\mathrm{e}^- \rightarrow 2\mathrm{H_2O} } $
- External Circuit:
Electrons flow from anode to cathode, generating electricity.
Byproducts: Water (and in some cycles, heat and small amounts of CO₂ if hydrocarbon fuels are reformed).
Detailed Explanation
The structure of a fuel cell involves several components that work together to generate electricity. At the anode, the fuel, typically hydrogen, is oxidized, producing electrons and positively charged ions. These electrons travel through an external circuit (thus generating electricity) while the ions move through the electrolyte to the cathode. At the cathode, the oxidant (usually oxygen) combines with the incoming ions and electrons to complete the cycle, producing water as a byproduct. It’s a continuous process as long as fuel and oxidant are supplied.
Examples & Analogies
Imagine a fuel cell as a factory assembly line. The anode is the starting point where raw materials (fuel) come in, and workers (electrons) are sent to do a job outside the factory (external circuit). At the end of the line (cathode), the finished product (water) is created by combining what came in with oxygen from the air.
Fuel Cell Thermodynamics
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Fuel cell performance and efficiency are governed by the principles of thermodynamics, particularly the first and second laws:
- Energy Conversion: Fuel cells directly convert chemical energy (from fuel) to electrical energy, bypassing the Carnot limit applicable to heat engines. Electrical output is determined by the change in Gibbs free energy (ΔG) of the cell reaction. Overall efficiency η is given by the ratio of usable electrical work to the enthalpy change (ΔH) of the reaction.
- Important Thermodynamic Quantities:
- Enthalpy change (ΔH): Total energy released by the reaction (both electrical and heat).
- Gibbs free energy (ΔG): Maximum electrical work obtainable under reversible (ideal) conditions at constant temperature and pressure.
- Cell Potential (E): E = -ΔG/nF, where n = number of electrons exchanged per mole of fuel and F = Faraday's constant.
- Efficiency: Theoretical (maximum) efficiency:
ηmax = ΔG/ΔH. For hydrogen fuel cells at standard conditions, this is around 83% (ΔH = 286 kJ/mol, ΔG = 237 kJ/mol).
Practical efficiency: Typically 40%-60% for most commercial stationary fuel cells, higher with combined heat and power (CHP) utilization. Actual operating voltage is lower than the reversible cell voltage due to practical (irreversible) losses (activation, ohmic, concentration). - Operating Conditions: Efficiency and output vary with temperature, pressure, and gas concentrations. High-temperature cells (MCFC, SOFC) have better fuel flexibility, internal reforming, and higher efficiency—at the cost of more complex material demands and start-ups.
- Advantages over Combustion Engines: Fuel cells are not heat engines; they can, in theory, exceed Carnot efficiency for direct chemical-electrical conversion. Waste heat can still be recovered for heating applications, further improving total energy utilization.
Detailed Explanation
Thermodynamics plays a crucial role in how fuel cells operate and their efficiency. Fuel cells directly convert fuel's chemical energy into electrical energy, unlike heat engines that operate under the Carnot limit, limiting their efficiency. Key thermodynamic principles involve understanding Gibbs free energy (ΔG), which indicates how much electrical work can be obtained. The efficiency calculations involve comparing the useful energy (ΔG) to the total energy released during the reaction (ΔH). While theoretically, hydrogen fuel cells can achieve efficiencies up to 83%, real-world applications typically see efficiencies between 40%-60%. Factors like temperature and pressure affect these efficiencies, and high-temperature cells like MCFC and SOFC offer better performance at the expense of added complexity.
Examples & Analogies
Think of thermodynamics in fuel cells like managing energy in a battery. The battery has a maximum capacity (ΔH), and how much energy you actually get out (ΔG) influences how much energy you can use. Just like you might have a battery that says it can provide 100 watts, but in practice, you only get 80 watts because of inefficiencies caused by how you use it, fuel cells experience similar challenges in real-world operations.
Key Concepts
-
Fuel Cells: Devices transforming chemical energy into electrical energy.
-
Electrolyte: Conducts ions, essential for fuel cell operation.
-
Classification: Primarily based on electrolyte type affecting performance.
-
Anode and Cathode: Key electrodes in energy conversion processes.
-
Thermodynamic Principles: Govern efficiency and performance of fuel cells.
Examples & Applications
A hydrogen fuel cell powering a car exhibits low emissions and high efficiency.
An SOFC can serve a local power grid with flexibility in fuel choices, running on natural gas or hydrogen.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Fuel cells generate with glee, energy from chemicals, just wait and see!
Stories
Once upon a time, in the land of clean energy, lived a hero called the Fuel Cell that transformed fuels like hydrogen into energy for vehicles and spaceships, leaving only water behind as a sign of its power.
Memory Tools
Anode - Attack, Cathode - Catch; Remember, ‘A’ for anode and ‘C’ for cathode to keep your cell working right!
Acronyms
PEMFC = Proton Exchange Membrane Fuel Cell, the friendly starter in the fuel cell family.
Flash Cards
Glossary
- Fuel Cell
An electrochemical device that converts chemical energy of fuel and oxidant directly into electricity, heat, and water.
- Electrolyte
A substance that conducts ions and allows the flow of electric charge in a fuel cell.
- Anode
The electrode where oxidation occurs, releasing electrons.
- Cathode
The electrode where reduction occurs, accepting electrons.
- Gibbs Free Energy (ΔG)
The maximum electrical work obtainable from a reaction at constant temperature and pressure.
- Enthalpy Change (ΔH)
The total change in energy during a reaction, inclusive of heat and work.
- Pelletized (PEMFC)
Proton Exchange Membrane Fuel Cell, known for low temperature operation and fast start-up.
- MCFC
Molten Carbonate Fuel Cell, operates at high temperatures for large power generation.
- SOFC
Solid Oxide Fuel Cell, operates at high temperatures with flexible fuel options.
Reference links
Supplementary resources to enhance your learning experience.
