Structure of Contractile Proteins
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Contractile Proteins
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will discuss contractile proteins, particularly actin and myosin. Can anyone tell me why these proteins are important for muscle function?

They help the muscles contract and relax, right?

Exactly! Contractile proteins are crucial for muscle contraction. Actin, which forms the thin filaments, is made of two strands of F-actin. Anyone knows what F-actin is made of?

F-actin is made up of G-actin, which are globular proteins.

Correct! G-actin monomers polymerize to form F-actin. Remember, you can think of 'G' for 'Globular' and 'F' for 'Filamentous' to keep track of these.

What about the thick filaments?

Great question! Myosin makes up the thick filaments, and it's composed of several meromyosins. Can anyone define what a meromyosin is?

Isn’t it the part of myosin that has a head and a tail?

Yes, the globular head is known as heavy meromyosin (HMM) while the tail is called light meromyosin (LMM). The head has ATPase activity and binding sites for actin, which is key for contraction.

So to summarize, actin filaments consist of F-actins and are supported by proteins like tropomyosin and troponin. Myosin filaments consist of meromyosins with HMM and LMM parts that allow muscle contraction.
Function of Contractile Proteins
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's discuss how these contractile proteins work together. Can anyone explain the role of tropomyosin?

Tropomyosin covers the binding sites on actin.

Precisely! In its relaxed state, tropomyosin blocks myosin from binding to actin. What happens during contraction?

Calcium ions must bind to troponin, and that moves tropomyosin away from the binding sites.

Great observation! This reveals the binding sites for myosin, forming cross-bridges. Now, let's discuss how energy is involved. What does the myosin head hydrolyze?

It hydrolyzes ATP for energy!

Exactly! The energy from ATP allows the myosin heads to pull the actin filaments towards the center of the sarcomere, causing contraction.

This sounds like the sliding filament theory!

Correct! The sliding filament theory explains that muscle contraction occurs as the thin filaments slide over thick filaments.

To summarize, during contraction, calcium binds to troponin, tropomyosin moves, and myosin heads use ATP to form cross-bridges and pull actin filaments.
Summary and Review
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's wrap up what we've learned about contractile proteins. What are the main components of the thin filament?

F-actin, tropomyosin, and troponin.

Great! And what about the thick filament?

It’s made up of myosin and meromyosins!

Exactly! Now, how do these proteins interact to cause contraction?

Calcium ions allow myosin to bind to actin and pull it, causing contraction.

Perfect! And importantly, we discussed how ATP is necessary for the myosin head to function effectively.

To summarize, the interaction between actin and myosin drives muscle contraction and involves ATP and calcium ions.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section highlights the composition of contractile proteins, including actin filaments with associated proteins like tropomyosin and troponin, and myosin filaments featuring meromyosin. Understanding these structures is crucial for grasping how muscle contraction occurs.
Detailed
Structure of Contractile Proteins
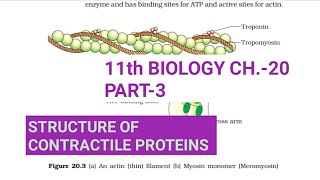
The structure of contractile proteins, specifically actin and myosin, is critical for muscle contraction. Actin, the thin filament, is composed of two helically-wound strands of F-actin, which is itself a polymer of monomeric G-actin. Surrounding the F-actin are two strands of tropomyosin, extending throughout its length. At intervals on these tropomyosin strands, a protein called troponin is found. In the resting state, a subunit of troponin blocks the active binding sites on actin for myosin.
On the other hand, myosin, the thick filament, consists of polymerized proteins known as meromyosins. Each meromyosin has a globular head and a tail. The head, referred to as heavy meromyosin (HMM), contains ATPase activity and binding sites for both ATP and actin. During muscle contraction, these proteins interact in a cycle that involves the sliding of actin over myosin, which is essential for muscle dynamics.
Youtube Videos



Audio Book
Dive deep into the subject with an immersive audiobook experience.
Composition of Actin Filaments
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
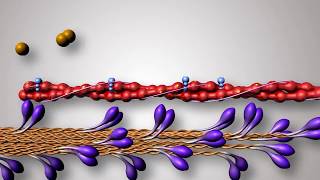
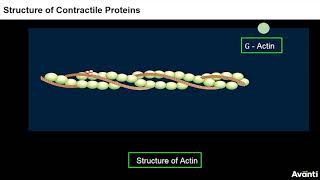
Each actin (thin) filament is made of two ‘F’ (filamentous) actins helically wound to each other. Each ‘F’ actin is a polymer of monomeric ‘G’ (Globular) actins. Two filaments of another protein, tropomyosin also run close to the ‘F’ actins throughout its length. A complex protein Troponin is distributed at regular intervals on the tropomyosin. In the resting state a subunit of troponin masks the active binding sites for myosin on the actin filaments.
Detailed Explanation
Actin filaments, which are crucial components in muscle contraction, are composed of two intertwined structures called F-actins. These F-actins themselves are made of smaller, globular units known as G-actins, which are polymerized to form the longer F-actin filament. Additionally, the tropomyosin protein runs alongside these actin filaments and plays a regulatory role. The troponin complex, associated with tropomyosin, periodically acts to cover the binding sites on actin for myosin, preventing premature muscle contraction until the muscle receives the appropriate signals.
Examples & Analogies
Think of actin as the flexible strands of a rope, where each strand (F-actin) is made of small beads (G-actins). The tropomyosin acts like a protective sheath that covers parts of the rope to prevent someone from grabbing it. When you want to pull the rope, a mechanism lifts the sheath (troponin), allowing people to grasp the rope securely and pull it toward them.
Myosin Structure Overview
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Each myosin (thick) filament is also a polymerised protein. Many monomeric proteins called Meromyosins constitute one thick filament. Each meromyosin has two important parts, a globular head with a short arm and a tail, the former being called the heavy meromyosin (HMM) and the latter, the light meromyosin (LMM). The HMM component, i.e.; the head and short arm projects outwards at regular distance and angle from each other from the surface of a polymerised myosin filament and is known as cross arm. The globular head is an active ATPase enzyme and has binding sites for ATP and active sites for actin.
Detailed Explanation
Myosin filaments are made up of multiple units known as meromyosins. Each of these units has two distinct parts: a globular head (heavy meromyosin) that is critical for the muscle's contractile function and a tail (light meromyosin) that helps to form the overall structure of the thick filament. This head contains enzymes and binding sites that interact with ATP, which provides the energy needed for muscular movement, as well as binding sites that connect with actin to facilitate contraction. The spatial orientation of these heads stems from their arrangement, forming what we recognize as the cross arms of myosin within muscles.
Examples & Analogies
Imagine myosin as a set of baseball players on a field. The players (meromyosins) form a long line (the thick filament), and each player has a unique role. The head of each player (heavy meromyosin) has the ability to throw the ball (bind to actin) and needs a ball (ATP) to throw effectively. When the entire team works together, they can move as one, similar to how muscle fibers contract when myosin heads pull on actin.
Key Concepts
-
Actin is a protein forming the thin filament crucial for muscle contraction.
-
Myosin is a protein forming the thick filament essential for muscle dynamics.
-
Tropomyosin and troponin regulate the binding of myosin to actin in muscle fibers.
-
Sliding filament theory explains how muscle contraction happens as actin slides past myosin.
Examples & Applications
In skeletal muscle, actin's structure allows it to interact dynamically with myosin, enabling contraction for movements like walking.
Cardiac and smooth muscles also utilize the same basic mechanisms of actin and myosin interaction for heartbeats and involuntary movements.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Actin thin, myosin thick, together they do the contraction trick!
Stories
Imagine actin and myosin as dancers in a tightly choreographed show. In the resting state, tropomyosin is like a curtain, hiding the stage, but when calcium joins the fun, the curtain lifts and they perform their act!
Memory Tools
Remember A-T-M: Actin, Tropomyosin, Myosin to recall key components in muscle contraction.
Acronyms
CATS
Calcium activates troponin
allowing actin and myosin to slide.
Flash Cards
Glossary
- Actin
A protein that forms thin filaments essential for muscle contraction.
- Myosin
A protein that forms thick filaments crucial for muscle contraction.
- Tropomyosin
A protein that blocks actin binding sites in its resting state.
- Troponin
A protein complex that interacts with tropomyosin and helps regulate muscle contraction.
- Meromyosin
The structural unit of myosin, which consists of heavy and light components.
- Crossbridge
The temporary connection formed when myosin heads bind to actin filaments.
- Sliding filament theory
A model explaining muscle contraction based on the sliding of actin over myosin.
Reference links
Supplementary resources to enhance your learning experience.
