Electron Transport System (ETS) and Oxidative Phosphorylation
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to the Electron Transport System (ETS)
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we'll explore the Electron Transport System, or ETS, which is crucial for energy production in cells. Can anyone tell me why the ETS is important in respiration?

Isn't it because it helps produce ATP?

Exactly! The ETS utilizes the electrons from NADH and FADH₂ to create a proton gradient that enables ATP synthesis. Let's remember this using the acronym 'ETS' - Energy Transfer System.

How does the ETS create this proton gradient?

Great question! The movement of electrons through the ETS complexes allows protons to be pumped across the membrane, creating a gradient. Remember this basic concept: 'Electrons pump Protons!'

So, what happens at the end of the ETS?

At the end, oxygen accepts electrons and combines with protons to form water. This is why we need oxygen for aerobic respiration. Remember, 'Oxygen Ends electrons'!

Got it! So, without oxygen, the entire process would halt, right?

Correct! The absence of oxygen prevents the electrons from flowing, and ATP production stops. Let's summarize: The ETS is vital for ATP production by transferring electrons and generating a proton gradient.
Components and Process of the ETS
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's delve deeper into the individual components of the ETS. Can someone name one of the complexes involved?

Is Complex I responsible for transferring electrons from NADH?

That's right! Complex I, also called NADH dehydrogenase, oxidizes NADH. Each complex has a specific role—understanding this helps us comprehend the workflow. Let's use the mnemonic 'NICE for Complexes' — Complex I for NADH, II for FADH₂, III for ubiquinone, and IV for cytochromes.

What about those protons? How are they pumped?

Protons are pumped across the membrane due to the energy released from the flow of electrons. This establishes a gradient that is critical for ATP synthesis at ATP synthase, the final complex.

So, Complex V is the ATP synthase?

Exactly! ATP synthase uses the proton gradient to convert ADP and inorganic phosphate into ATP. Remember, 'A Strong Proton Flow = A Strong ATP Flow'!

What about FADH₂? Does it make less ATP than NADH?

Yes, because FADH₂ enters the chain at Complex II, producing approximately two ATP molecules, while NADH produces around three. Summarizing: 'More NADH, More ATP!'
Oxidative Phosphorylation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's focus on oxidative phosphorylation. Why do we call it that?

Is it because ATP is synthesized through oxidation reactions?

Yes! In oxidative phosphorylation, the oxidation of electron carriers phosphorylates ADP to form ATP. Think of it as 'Oxy-ATP'! What role does oxygen play here?

Oxygen acts as the final electron acceptor!

Correct! This is essential because it removes electrons and protons, preventing a backlog in the ETS. Without oxygen: 'No End, No Energy'.

What happens if there’s no oxygen?

In its absence, cells must resort to anaerobic pathways, drastically reducing ATP yield. Thus, we summarize, 'Oxygen is the Key for Better Energy!'

How many ATP can we make from one glucose molecule with the entire process?

The theoretical maximum is 38 ATP but remember that some energy is lost in the process. Always remember this as the '38 club!'
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The Electron Transport System (ETS) transfers electrons from NADH and FADH₂ through a series of protein complexes in the inner mitochondrial membrane, ultimately forming water and facilitating ATP synthesis via oxidative phosphorylation. This part of aerobic respiration is vital for energy production in cells.
Detailed
Detailed Summary
The Electron Transport System (ETS) is a crucial component of aerobic respiration, taking place in the inner mitochondrial membrane. It facilitates the transfer of electrons that are derived from NADH and FADH₂ generated in earlier metabolic pathways, specifically the Krebs cycle. As electrons move through the ETS, they pass from one electron carrier to another, resulting in the release of energy.
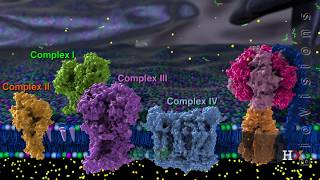
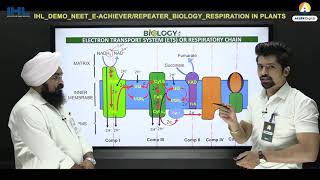
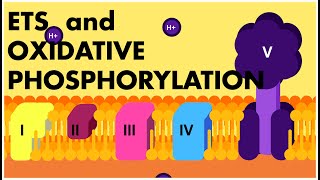
The ETS is organized into several complexes:
- Complex I: NADH dehydrogenase oxidizes NADH to NAD⁺ and transports electrons to ubiquinone.
- Complex II: FADH₂ is oxidized to FAD, also transferring electrons to ubiquinone.
- Complex III: Ubiquinol (reduced ubiquinone) transfers electrons to cytochrome c.
- Complex IV: Cytochrome c oxidase passes electrons to molecular oxygen, reducing it to water.
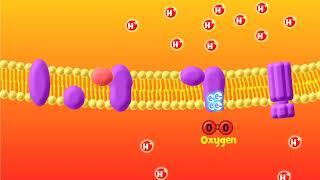
During this electron transport, a proton gradient is created across the inner mitochondrial membrane, which is essential for ATP synthesis via ATP synthase (Complex V). For each NADH oxidized, approximately three ATP molecules are produced, while FADH₂ yields around two ATP molecules. Oxygen acts as the final electron acceptor, making it indispensable for the aerobic respiration process. Overall, the coupling of electron transport and proton gradient formation leads to oxidative phosphorylation, a critical step for energy production in aerobic organisms.
Youtube Videos

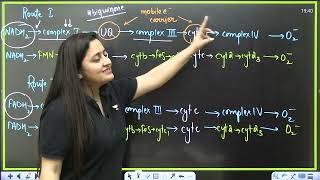




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Overview of ETS
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The following steps in the respiratory process are to release and utilise the energy stored in NADH+H+ and FADH2. This is accomplished when they are oxidised through the electron transport system and the electrons are passed on to O2 resulting in the formation of H2O.
Detailed Explanation
The Electron Transport System (ETS) is a series of protein complexes located in the inner membrane of mitochondria. This system is essential for aerobic respiration because it facilitates the transfer of electrons derived from NADH and FADH2. When these molecules are oxidised, they release energy that is used to pump protons across the mitochondrial membrane, creating a proton gradient. This process culminates in the reduction of oxygen to water, which is crucial for cellular respiration.
Examples & Analogies
Think of ETS like a water wheel in a river. As water (electrons) flows over the wheel (protein complexes), it spins and generates energy (ATP). The more powerful the flow, the more energy you can spin out of it. Without water (oxygen), the wheel won't turn, and energy production comes to a standstill.
Components of the Electron Transport Chain
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The metabolic pathway through which the electron passes from one carrier to another, is called the electron transport system (ETS) and it is present in the inner mitochondrial membrane. Electrons from NADH produced in the mitochondrial matrix during citric acid cycle are oxidised by an NADH dehydrogenase (complex I), and electrons are then transferred to ubiquinone located within the inner membrane.
Detailed Explanation
In the electron transport chain, electrons move through a series of complexes (I to IV). Each complex has a specific role: Complex I receives electrons from NADH, while Complex II receives them from FADH2. Ubiquinone (also known as Coenzyme Q) acts as a mobile carrier that shuttles the electrons from Complex I and II to Complex III. This movement helps to generate an electrochemical gradient that is necessary for ATP synthesis.
Examples & Analogies
Imagine a relay race where each runner (electron carrier) passes the baton (electron) down the line. As each runner passes the baton, they gain momentum and energy (proton gradient), which is used at the end of the race to power a finishing burst (ATP production).
Role of Oxygen and ATP Synthesis
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When the electrons pass from one carrier to another via complex I to IV in the electron transport chain, they are coupled to ATP synthase (complex V) for the production of ATP from ADP and inorganic phosphate. The number of ATP molecules synthesised depends on the nature of the electron donor.
Detailed Explanation
Oxygen plays a critical role as the final electron acceptor in the electron transport chain. When electrons move through Complexes I to IV, they eventually reduce oxygen, forming water. This reduction allows for the continuation of electron flow through the chain. The energy released during these transfers powers ATP synthase, which synthesizes ATP from ADP and inorganic phosphate. Each NADH results in the production of about 3 ATP, while FADH2 yields around 2 ATP.
Examples & Analogies
Think of ATP synthesis as a water pump. The movement of water (electrons) turns the pump (ATP synthase), which fills a reservoir (ATP) that can be used whenever energy is needed. Without a steady flow of water (oxygen), the pump cannot function, and the reservoir cannot fill.
Oxidative Phosphorylation
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Although the aerobic process of respiration takes place only in the presence of oxygen, the role of oxygen is limited to the terminal stage of the process. Yet, the presence of oxygen is vital, since it drives the whole process by removing hydrogen from the system.
Detailed Explanation
Oxidative phosphorylation refers to the generation of ATP as a result of electron transport and the use of oxygen as the final electron acceptor. This process is essential because it allows cells to produce a large quantity of ATP efficiently. Oxygen's role is crucial, as it ensures that the electrons continue to flow through the transport chain, and without it, the entire energy production process would halt.
Examples & Analogies
Consider oxidative phosphorylation as a factory assembly line. The workers (electrons) move along the line, but to keep operations running smoothly and prevent overcrowding (buildup of electrons), the final worker must clear the line (oxygen). Once the line is clear, production (ATP creation) can continue at a high rate.
Key Concepts
-
Electron Transport Chain: A series of complexes where electrons are transported, producing a proton gradient for ATP synthesis.
-
Oxygen's Role: Acts as the final electron acceptor in the ETS, leading to water formation.
-
Proton Gradient: Created by the pumping of protons through the mitochondrial membrane during electron transport, driving ATP synthesis.
Examples & Applications
During aerobic respiration, the electrons from NADH and FADH₂ are sent through the ETS leading to ATP production.
In the absence of oxygen, cells switch to fermentation, which produces much less ATP.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In the MITOCHONDRIA so grand, / Electrons take a stand. / Through complexes they race, / Making ATP's space!
Stories
Once upon a time in the mitochondria, electrons rode a rollercoaster through complexes. They created a proton party, helping ATP to come to life, all thanks to Oxygen, the hero who saved them from darkness!
Memory Tools
To remember the ETS order: C1 (Complex I), C2 (Complex II), C3 (Complex III), C4 (Complex IV) — 'I Like To Causally 4 ATP!'
Acronyms
ETS
Electron Transport System - Energy Transfer through Sequential reactions.
Flash Cards
Glossary
- Electron Transport System (ETS)
A series of protein complexes in the inner mitochondrial membrane that transfer electrons from NADH and FADH₂ to oxygen, facilitating ATP synthesis.
- Oxidative Phosphorylation
The process of ATP production resulting from the transfer of electrons through the electron transport chain and the formation of a proton gradient.
- Proton Gradient
The difference in proton concentration across the mitochondrial membrane, essential for driving ATP synthesis.
- NADH and FADH₂
NADH is the reduced form of nicotinamide adenine dinucleotide, while FADH₂ is the reduced form of flavin adenine dinucleotide—both are electron carriers produced during metabolic processes.
- ATP Synthase
An enzyme complex that synthesizes ATP from ADP and inorganic phosphate by utilizing the proton gradient created by the ETS.
Reference links
Supplementary resources to enhance your learning experience.
