Respiratory Quotient
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Respiratory Quotient
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Good morning, class! Today we're diving into the concept of the Respiratory Quotient, or RQ. Can anyone tell me what RQ is?

Isn't it a ratio of CO2 produced to O2 consumed during respiration?

Exactly, Student_1! The formula is RQ equals the volume of CO2 evolved divided by the volume of O2 consumed. It's important because it gives us insight into what type of substrate is being used for respiration.

So does that mean the RQ changes with different substrates?

Yes, it does! For example, if carbohydrates are being metabolized, the RQ equals 1, indicating that equal amounts of O2 and CO2 are exchanged.

What happens with fats and proteins?

With fats, the RQ is less than 1. For proteins, it tends to be around 0.9. These differences help us understand the metabolism of different organisms.

Can you give us an example?

Sure! When glucose is oxidized, the reaction is: C6H12O6 + 6O2 → 6CO2 + 6H2O. Thus, the RQ = 6CO2/6O2, which equals 1. Let's recap: the RQ changes according to the type of respiratory substrate used.
Impact of Substrates on RQ
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's delve deeper into how different substrates affect the RQ. For instance, what happens when fats are used as the primary energy source?

The RQ would be less than 1, right?

Correct! The RQ for fats can be as low as 0.7. This means that when fats undergo respiration, less CO2 is produced for every O2 consumed, indicating that fats release more energy per molecule than carbohydrates.

So, does that mean fats are more efficient?

That's one way to look at it! Fats contain more carbon atoms than carbohydrates, resulting in more energy when they're oxidized.

How about proteins?

Proteins have an RQ of around 0.9. They are metabolized differently than fats and carbohydrates, contributing to RQ variations.

This is fascinating! So we see different energy patterns through RQ.

Absolutely! Let's summarize what we learned. RQ varies based on respiratory substrates, which aids our understanding of metabolism efficiency.
Calculating RQ
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's practice calculating RQ. If we have a fatty acid like tripalmitin oxidizing, can anyone help with the calculation?

We have 2 molecules of C51H98O6 oxidizing with 145 O2 to form 102 CO2 and 98 H2O.

Great! So what would the RQ be?

The RQ would be 102 CO2 over 145 O2, which is around 0.7!

Exactly! Now, think about why this is important.

It helps understand how living organisms utilize different fuels.

Right again! RQ not only reveals metabolic choices but also reflects an organism's energy needs. What about glucose or carbohydrates?

That's RQ 1, showing equal exchange!

Perfect! Always remember how different substrates influence RQ and what it tells us about metabolic processes.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The respiratory quotient is the ratio of carbon dioxide produced to oxygen consumed in respiration, reflecting the type of respiratory substrate. Carbohydrates yield an RQ of 1, while proteins and fats provide different RQ values, revealing insights into metabolic processes.
Detailed
Respiratory Quotient (RQ)
The respiratory quotient (RQ), also known as the respiratory ratio, is a crucial concept in understanding the efficiency and type of metabolism occurring within an organism. It is defined mathematically as:
$$ RQ = \frac{\text{Volume of } CO_2 \text{ evolved}}{\text{Volume of } O_2 \text{ consumed}} $$
The value of RQ varies depending on the substrate used in respiration:
- For Carbohydrates: When carbohydrates are oxidized completely, the RQ equals 1.
Example Reaction:
$$ C_6H_{12}O_6 + 6O_2 \rightarrow 6CO_2 + 6H_2O + \text{Energy} $$
This results in:
$$ RQ = \frac{6CO_2}{6O_2} = 1.0 $$
- For Fats: The RQ is less than 1.
For instance, when tripalmitin (a fatty acid) is oxidized:
$$ 2(C_{51}H_{98}O_6) + 145 O_2 \rightarrow 102 CO_2 + 98 H_2O + \text{Energy} $$
The calculation yields:
$$ RQ = \frac{102 CO_2}{145 O_2} \approx 0.7 $$
- For Proteins: The RQ for protein metabolism typically hovers around 0.9.
Recognizing that living organisms do not exclusively utilize one substrate is essential; they metabolize a combination of carbohydrates, proteins, and fats, which results in variable RQ values during respiration. Understanding RQ allows for insights into the metabolic state and efficiency of organisms in different physiological conditions.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Respiratory Quotient (RQ)
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
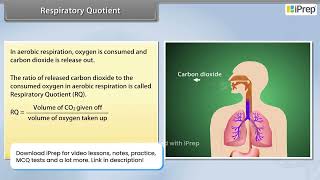
Let us now look at another aspect of respiration. As you know, during aerobic respiration, O is consumed and CO is released. The ratio of the volume of CO evolved to the volume of O consumed in respiration is called the respiratory quotient (RQ) or respiratory ratio.
RQ = volume of CO evolved / volume of O consumed
Detailed Explanation
The respiratory quotient (RQ) is a crucial concept in understanding cellular respiration. It measures the efficiency and type of substrate used in the respiration process of an organism. Specifically, the RQ is calculated by dividing the amount of carbon dioxide (CO2) produced by the amount of oxygen (O2) consumed during respiration. This ratio can help reveal whether the organism is primarily burning carbohydrates, fats, or proteins as energy sources.
Examples & Analogies
Imagine you are observing a car. If it runs smoothly without any issues, you can infer that it is using fuel efficiently. Similarly, RQ indicates how well an organism is using its energy sources. A higher RQ suggests that carbohydrates are being predominantly used, much like a car running on high-quality gasoline.
RQ for Carbohydrates
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When carbohydrates are used as substrate and are completely oxidised, the RQ will be 1, because equal amounts of CO and O are evolved and consumed, respectively, as shown in the equation below :
C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy
RQ = 6CO2 / 6O2 = 1.0
Detailed Explanation
When carbohydrates such as glucose are fully oxidized during aerobic respiration, the amount of carbon dioxide produced is equal to the amount of oxygen consumed. Thus, for carbohydrates, the RQ value is 1. This is significant because it indicates a balanced use of energy, where every molecule of O2 is providing energy that is exactly matched by the amount of CO2 produced.
Examples & Analogies
Consider baking a loaf of bread. When the dough rises (a sign of fermentation), it uses sugar (a carbohydrate) and releases carbon dioxide—like the process in cellular respiration. Just like a well-risen loaf has balanced ingredients, a balanced RQ of 1 indicates efficient energy use from carbohydrates.
RQ for Fats
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When fats are used in respiration, the RQ is less than 1. Calculations for a fatty acid, tripalmitin, if used as a substrate is shown:
2(C51H98O6) + 145O2 → 102CO2 + 98H2O + energy
RQ = 102CO2 / 145O2 = 0.7
Detailed Explanation
Fats yield less carbon dioxide per unit of oxygen consumed compared to carbohydrates, resulting in an RQ value of less than 1. For example, when tripalmitin, a type of fat, is oxidized, only 0.7 liters of CO2 is produced for every liter of O2 consumed. This indicates that fats require more oxygen to be fully oxidized, reflecting their higher energy density.
Examples & Analogies
Think of a long road trip in a fuel-efficient car versus a gas guzzler. The gas guzzler uses more fuel (oxygen) to go the same distance (generate energy), much like how fat metabolism consumes more oxygen and produces less CO2 than carbohydrate metabolism.
RQ for Proteins
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When proteins are respiratory substrates the ratio would be about 0.9.
Detailed Explanation
Proteins, when used as respiratory substrates, give an RQ value around 0.9. This suggests that while they are relatively efficient for energy production, they are not as efficient as carbohydrates or less so than fats, meaning proteins also have a significant energy yield but require a certain amount of oxygen to produce energy, similar to both carbohydrates and fats.
Examples & Analogies
Consider a diverse meal with protein, carbs, and fats. Each type offers energy but does so at different rates and efficiencies, akin to different fuels for different engines. Just as an engine might be tuned for different fuels, our bodies adjust RQ based on the energy sources available.
Understanding Mixed Substrates
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
What is important to recognise is that in living organisms respiratory substrates are often more than one; pure proteins or fats are never used as respiratory substrates.
Detailed Explanation
In nature, organisms typically do not rely purely on one type of substrate—like only carbohydrates or only fats—for respiration. Instead, they use a mixture of substrates depending on availability and metabolic needs. This blend leads to varied RQ values that can change based on diet or activity level, indicating the versatility of living systems in energy metabolism.
Examples & Analogies
Imagine a buffet with various food types—carbs, proteins, and fats. Diners will choose a mix based on their preferences and needs, leading to varied energy outputs. Similarly, our bodies adapt to available fuels, changing how we metabolize and release energy.
Key Concepts
-
Respiratory Quotient (RQ): Ratio of CO2 produced to O2 consumed.
-
Fats and Proteins: Have varying RQ values, indicating different metabolic efficiencies.
-
Energy Release: Different substrates release energy differently based on their molecular structure.
Examples & Applications
Carbohydrates metabolized: C6H12O6 + 6O2 → 6CO2 + 6H2O, RQ = 1.
Tripalmitin metabolized: 2(C51H98O6) + 145O2 → 102CO2 + 98H2O, RQ = 0.7.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
RQ tells us what we respire, carbs, proteins, fats, it'll inspire.
Stories
Imagine a race with runners: carbohydrates run straight with 1.0, fats sneakily glide at 0.7, and proteins jog in between at 0.9.
Memory Tools
C for Carbs = RQ 1, F for Fats = RQ less than 1, P for Proteins = RQ 0.9.
Acronyms
Carbs - 1.0, Fats - <1, Proteins - ~0.9 (C-F-P mnemonic).
Flash Cards
Glossary
- Respiratory Quotient (RQ)
The ratio of the volume of CO2 evolved to the volume of O2 consumed during respiration.
- Respiratory Substrate
The organic molecule that is oxidized to release energy during respiration.
- Aerobic Respiration
A form of respiration that involves the use of oxygen to completely oxidize substrates.
- Anaerobic Respiration
A form of respiration that occurs without oxygen, often resulting in fermentation.
Reference links
Supplementary resources to enhance your learning experience.
