Fermentation
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Fermentation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will discuss the process of fermentation. Can anyone tell me what fermentation is?

Is it how yeast makes alcohol?

Exactly! Fermentation is a process where microorganisms like yeast convert sugars into alcohol and carbon dioxide in the absence of oxygen. This process also happens in our muscles during intense exercise when oxygen is scarce.

So, it's like an alternate way to produce energy?

Correct! It's an anaerobic process that allows cells to generate a small amount of energy, especially when oxygen is not available. But, unlike aerobic respiration, fermentation is much less efficient.

What happens to the pyruvic acid during fermentation?

Great question! Pyruvic acid can be converted to either ethanol and CO2 in yeasts or to lactic acid in animal cells. This conversion allows the regeneration of NAD+, which is necessary for glycolysis to continue.

In summary, fermentation is vital for energy production in low oxygen situations, but remember, it provides less energy compared to aerobic pathways.
Lactic Acid vs. Alcohol Fermentation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's dive deeper into the two main types of fermentation: lactic acid fermentation and alcoholic fermentation. Who can explain lactic acid fermentation?

I think it happens in our muscles when we run out of oxygen.

Exactly! During strenuous exercise, our muscle cells can convert pyruvic acid to lactic acid when oxygen is not available, which uses lactate dehydrogenase in the process.

And what about alcoholic fermentation?

In alcoholic fermentation, yeasts convert pyruvic acid to ethanol and carbon dioxide through a two-step process involving pyruvic acid decarboxylase and alcohol dehydrogenase. This is how we produce beer and wine!

So, in both processes, energy is released, right?

Yes, but remember, the energy yield from fermentation is quite low—only about 2 ATP molecules per glucose molecule. This compares to up to 38 ATP during aerobic respiration.

To summarize, lactic acid fermentation produces lactic acid while alcoholic fermentation yields ethanol and CO2, both of which are crucial for energy in anaerobic conditions.
Energy Yield and Toxicity of Byproducts
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand the processes, can someone tell me about the energy yield in fermentation?

It’s much lower than in aerobic respiration, right?

Exactly! In fermentation, we gain just 2 ATP molecules from the breakdown of each glucose molecule compared to up to 38 in aerobic respiration. This makes fermentation a last-resort energy process.

But why is that? Are there any dangers in the byproducts?

Great observation! The byproducts of fermentation, especially lactic acid or ethanol, can become toxic at high concentrations. For example, yeasts die when alcohol levels exceed around 13%.

So, fermentation is a temporary solution to energy production?

Exactly! It allows cells to survive in low oxygen environments but is not sustainable for long periods. It’s great for short bursts of energy but comes with its own challenges!

In summary, while fermentation allows energy production, it is much less efficient and creates potentially toxic byproducts.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In fermentation, organisms such as yeast convert pyruvic acid into ethanol and carbon dioxide, while animals may produce lactic acid from pyruvic acid under oxygen-limited conditions. Both processes yield less energy compared to aerobic respiration, with inadequate ATP production and byproducts that can be harmful if accumulated.
Detailed
Detailed Summary of Fermentation
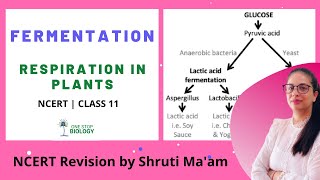
Fermentation is a biochemical process that occurs anaerobically, meaning it happens in the absence of oxygen. During this process, glucose undergoes partial oxidation to produce energy in the form of ATP, albeit significantly less than that produced through aerobic respiration. The key initial substrate in fermentation is pyruvic acid, which is generated from glycolysis, the breakdown of glucose.
In yeasts, for example, fermentation converts pyruvic acid into carbon dioxide (CO2) and ethanol through the action of specific enzymes such as pyruvic acid decarboxylase and alcohol dehydrogenase. During this process, NADH is oxidized back to NAD+, which is crucial for sustaining glycolysis so that energy can continue to be produced, even in the absence of oxygen.
In animal cells, particularly muscle cells during intense exercise, pyruvic acid is converted into lactic acid through the action of lactate dehydrogenase. This conversion allows the regeneration of NAD+ but can lead to an accumulation of lactic acid, resulting in muscle fatigue.
Overall, fermentation is characterized by the following points:
- It leads to a much lower energy yield compared to aerobic respiration—less than 7% of the energy in glucose is captured as ATP.
- The byproducts of fermentation (alcohol or lactic acid) can be toxic to the organisms producing them if they accumulate beyond certain levels—in yeasts, for instance, alcohol concentrations exceeding approximately 13% are lethal.
Thus, while fermentation serves as a critical energy-producing process under anaerobic conditions, it is less efficient than aerobic respiration and produces byproducts that can inhibit further cellular processes.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Overview of Fermentation
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In fermentation, say by yeast, the incomplete oxidation of glucose is achieved under anaerobic conditions by sets of reactions where pyruvic acid is converted to CO2 and ethanol. The enzymes, pyruvic acid decarboxylase and alcohol dehydrogenase catalyse these reactions. Other organisms like some bacteria produce lactic acid from pyruvic acid.
Detailed Explanation
Fermentation is a process that takes place without oxygen (anaerobic) where glucose is not fully oxidized. In yeast, a type of fungus, the conversion of glucose to ethanol and carbon dioxide occurs when pyruvic acid breaks down during the fermentation process. Key enzymes like pyruvic acid decarboxylase and alcohol dehydrogenase facilitate these transformations. This same pyruvic acid can lead to lactic acid when processed by certain bacteria or in the muscles during intense exercise when oxygen is low.
Examples & Analogies
Think of fermentation like making bread. When you mix flour (glucose) with yeast (which ferments the glucose), the yeast produces carbon dioxide (the gas that makes the bread rise) and ethanol (which evaporates during baking). This is similar to how the yeast creates alcohol in beverages or even how our muscles create lactic acid when we exercise hard.
Energy Yield from Fermentation
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In both lactic acid and alcohol fermentation not much energy is released; less than seven percent of the energy in glucose is released and not all of it is trapped as high energy bonds of ATP. Also, the processes are hazardous – either acid or alcohol is produced.
Detailed Explanation
Fermentation is not an efficient process for energy production compared to aerobic respiration. It only captures about 7% of the energy that was stored in the glucose molecule, resulting in the creation of products like lactic acid or ethanol, which can be harmful in high concentrations. This inefficiency is one reason why organisms prefer aerobic processes when oxygen is available, as they can extract far more energy.
Examples & Analogies
Imagine trying to power a car with just a few drops of fuel, resulting in a very short trip. That’s similar to how fermentation works—it can “run” but not very effectively. On the other hand, using gasoline (aerobic respiration) allows for a long, fruitful drive.
Net ATP Production in Fermentation
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
What is the net ATPs that is synthesised (calculate how many ATP are synthesised and deduct the number of ATP utilised during glycolysis) when one molecule of glucose is fermented to alcohol or lactic acid?
Detailed Explanation
When glucose undergoes fermentation, it first goes through glycolysis where 2 ATP molecules are consumed and 4 ATP are generated, resulting in a net yield of 2 ATP molecules. However, fermentation itself does not produce additional ATP. Thus, regardless of whether it results in alcohol or lactic acid, the total ATP production from one glucose molecule during fermentation remains at 2, compared to the much higher yields in aerobic conditions.
Examples & Analogies
Think of a student preparing for exams by studying late at night. They put in lots of effort (energy spent on studying, like ATP consumed) but only gain a limited understanding (only 2 ATP gained), much like glucose turning into lactic acid or ethanol through fermentation.
Consequences of Alcoholic Fermentation
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Yeasts poison themselves to death when the concentration of alcohol reaches about 13 percent. What would be the maximum concentration of alcohol in beverages that are naturally fermented?
Detailed Explanation
In yeast fermentation, alcohol is the byproduct of glucose breakdown. However, as the alcohol concentration increases, it eventually becomes toxic to the yeast itself; typically, this becomes lethal around a concentration of 13%. Therefore, naturally fermented beverages cannot exceed this concentration without killing the yeast involved in their production.
Examples & Analogies
This is similar to how some plants can only absorb a certain amount of water before the roots start to rot. In both cases, there's a limit to what can be tolerated; for yeast, it's the alcohol concentration, while for the plant, it's the water levels.
Complete Oxidation versus Fermentation
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
What then is the process by which organisms can carry out complete oxidation of glucose and extract the energy stored to synthesise a larger number of ATP molecules needed for cellular metabolism?
Detailed Explanation
The process that allows for complete oxidation of glucose is aerobic respiration, which involves multiple steps including glycolysis, the Krebs cycle, and the electron transport chain. This sequence effectively breaks down glucose all the way to carbon dioxide and water, yielding up to 38 ATP molecules from one glucose molecule, which is significantly more than what fermentation can offer.
Examples & Analogies
Think of aerobic respiration as a well-planned road trip with multiple fuel stops allowing you to travel far and efficiently, versus fermentation, which is like a quick errand that drains your resources fast, giving you only a little return before you run out of gas.
Key Concepts
-
Fermentation: Anaerobic process converting glucose into energy, yielding ethanol or lactic acid.
-
Energy Yield: Fermentation is inefficient compared to aerobic respiration, resulting in only 2 ATP molecules per glucose molecule.
-
Byproducts: Fermentation produces toxic compounds like ethanol or lactic acid that can inhibit further metabolism.
Examples & Applications
Yeast fermentation produces beer and bread by converting sugars into carbon dioxide and ethanol.
Muscle cells convert pyruvic acid into lactic acid during intense exercise, causing muscle fatigue.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Fermentation, without air's flow, makes alcohol, the drink we know.
Stories
Imagine a bakery where yeast eats sugar and breathes out bubbles. Those bubbles make our bread rise while the alcohol evaporates!
Memory Tools
A for Alcohol - Yeast, L for Lactic - Muscle, F for Fermentation - Energy!
Acronyms
FLAP
Fermentation Leads to Alcohol or Pyruvate (for lactic acid).
Flash Cards
Glossary
- Fermentation
A biochemical process that converts sugars to acids, gases, or alcohol in the absence of oxygen.
- Pyruvic Acid
A three-carbon compound that is a key intermediate in several metabolic pathways.
- Lactic Acid
A byproduct of lactic acid fermentation, produced from pyruvic acid when oxygen is scarce.
- Ethanol
An alcohol produced from the fermentation of sugars, often used in alcoholic beverages.
- NAD+
Nicotinamide adenine dinucleotide, a coenzyme involved in redox reactions, critical for energy metabolism.
Reference links
Supplementary resources to enhance your learning experience.
