Competitive Electron Transfer Reactions
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Redox Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are diving into redox reactions, specifically focusing on competitive electron transfer. Can anyone explain what redox means?

I think redox refers to reactions where one species gets oxidized while another gets reduced.

Exactly! And it's all about the transfer of electrons. When one substance loses electrons, another gains them. This is fundamental to understanding reactions like zinc in copper nitrate. Can anyone tell me what happens when zinc is placed in copper nitrate?

The zinc strip is coated with copper, and the blue color of the solution disappears!

Great observation! So, we see oxidation of zinc and reduction of copper ions. Remember this: 'Oxidation is loss, Reduction is gain' — you can use the mnemonic OIL RIG to remember this. Let's discuss the implications of electron competition next.
Electron Competition
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's talk about how metals compete for electrons. What can we infer from the reactions of zinc and copper?

Zinc is more reactive than copper, so it can displace copper from its solution, right?

Correct! Reactions suggest an order of electron-releasing tendencies. Zinc will always lose electrons to copper. Can you guess the order of electron release tendencies among metals?

I think it goes Zn > Cu > Ag, based on what we discussed about their competitions!

Exactly! That hierarchy is essential in constructing a metal activity series. Remember: more reactive metals can reduce less reactive metal ions. Now, how does this relate to the electrochemical processes we'll study later?
Implications of Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we've covered the basics, let's explore how this concept extends to real-world applications, such as batteries and corrosion.

So, does this mean the principles we discussed can also apply to how batteries generate electricity?

Absolutely! In galvanic cells, we have a similar competitive electron transfer at work. Every element's tendency to either be oxidized or reduced helps determine how the cell operates. Can you recall how we observed these processes in the lab?

We watched zinc and copper reactions and how they changed the solution!

Perfect! This understanding is vital for developing electrochemical cells in industry. Remember, competitive reactions are the foundation of how chemical energy is transformed into electrical energy.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section discusses the interaction between metals and their ions in aqueous solutions, exemplified by the reaction of zinc with copper nitrate, and explores the implications of electron transfer and competition among metals for reduction. The concepts established aid in understanding more complex electrochemical processes.
Detailed
Competitive Electron Transfer Reactions
This section examines the fundamental principles of competitive electron transfer reactions, particularly focusing on the interactions between metallic zinc and aqueous copper nitrate. It illustrates how the zinc strip, when placed in the copper nitrate solution, undergoes oxidation to form Zn²⁺ ions, while copper(II) ions are reduced to metallic copper, resulting in a change of color in the solution from blue to colorless.
Key Points:
- Redox Principle: Zinc loses electrons and is oxidized to Zn²⁺, while copper ions gain electrons and are reduced to Cu. This is a hallmark of redox reactions where oxidation and reduction occur simultaneously.
- Equilibrium Considerations: When the reaction is reversed by placing copper into a zinc sulfate solution, no significant reaction occurs, highlighting the favorability of the initial zinc-to-copper transfer reaction.
- Comparative Electron Release: The section underlines a competition for electrons among metals; the electron releasing tendency is illustrated with the hierarchy: Zn > Cu > Ag. This establishes the concept of a metal activity series important for predicting the outcomes in redox reactions.
In summary, the reactions not only illustrate competitive electron transfer but also serve as the foundation for understanding electrochemical cells and redox behavior in various contexts.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Reaction Between Zinc and Copper Nitrate
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
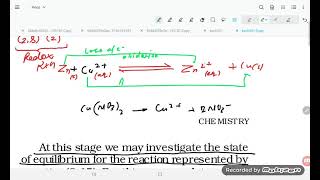
Place a strip of metallic zinc in an aqueous solution of copper nitrate as shown in Fig. 7.1, for about one hour. You may notice that the strip becomes coated with reddish metallic copper and the blue colour of the solution disappears.
Detailed Explanation
When a strip of zinc is placed in copper nitrate solution, a chemical reaction occurs. Zinc, being more reactive than copper, displaces copper ions from the solution. As zinc oxidizes, it loses electrons and transforms into Zn²⁺ ions, while copper ions (Cu²⁺) in the solution gain those electrons and get reduced to solid copper, which deposits on the zinc strip. The blue color is due to Cu²⁺ ions; its disappearance indicates their conversion to solid copper.
Examples & Analogies
Imagine zinc as a bully who pushes copper out of a playground. While zinc gets more powerful (becomes Zn²⁺), copper turns into a shy kid who blends back into the background (becomes solid copper). This reaction demonstrates how more reactive metals can take the place of less reactive ones.
Formation of Zinc Sulphide
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Formation of Zn2+ ions among the products can easily be judged when the blue colour of the solution due to Cu2+ has disappeared. If hydrogen sulphide gas is passed through the colourless solution containing Zn2+ ions, appearance of white zinc sulphide, ZnS can be seen on making the solution alkaline with ammonia.
Detailed Explanation
After the reaction between zinc and copper nitrate, when the blue color fades, it signifies that zinc ions (Zn²⁺) have formed. If hydrogen sulfide gas (H₂S) is introduced into this solution, it reacts with Zn²⁺ ions to form a white precipitate of zinc sulfide (ZnS) when the solution is turned alkaline using ammonia. The reaction can be represented as Zn²⁺ + H₂S → ZnS (solid) + 2H⁺. This highlights the ability to confirm the presence of zinc ions in the solution using chemical reactions.
Examples & Analogies
Think of hydrogen sulfide as a detective that reveals the hidden identity of zinc ions. When it arrives and interacts with the invisible zinc ions in the solution, it causes them to become visible as a white solid (ZnS) that you can see, just like finding a hidden clue in a mystery.
Investigating the State of Equilibrium
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
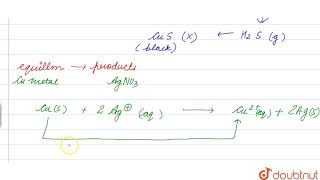
At this stage we may investigate the state of equilibrium for the reaction represented by equation (7.15). For this purpose, let us place a strip of metallic copper in a zinc sulphate solution. No visible reaction is noticed...
Detailed Explanation
Here we test copper in zinc sulfate solution to observe equilibrium. When copper is added to the zinc sulfate solution, no visible reaction occurs, meaning that the copper ions (Cu²⁺) cannot displace zinc because zinc is more reactive. The conclusion drawn from this experiment is that in this system, the products (Cu²⁺ and Zn) are favored over the reactants because the properties and reactivity maintain a specific equilibrium.
Examples & Analogies
Consider this situation like a sports game where the team that consistently wins (zinc) is facing off against a weaker team (copper). Even if copper tries to score by being added into the game, it can't replace the team because zinc's winning streak keeps it firmly in the league—this represents the equilibrium state.
Electron Transfer Between Copper and Silver
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Let us extend electron transfer reaction now to copper metal and silver nitrate solution in water...
Detailed Explanation
In this example, when copper reacts with silver nitrate solution, copper donates electrons to silver ions, reducing them to silver metal (Ag). During this process, copper oxidizes to Cu²⁺ ions. This demonstrates another type of competitive reaction where reactivity plays a crucial role between metals, showing the hierarchy in electron transfer based on their ability to donate or accept electrons.
Examples & Analogies
Imagine a friendly competition where teammates (copper) are assisting their friends (silver ions) by giving them energy (electrons) to score (reduce to solid silver). At the same time, the teammates get tired (oxidized to Cu²⁺ ions) but help their friends shine by transforming them into solid silver.
Key Concepts
-
Zinc and copper demonstrate competitive electron transfer.
-
Electron transfer in redox reactions occurs simultaneously.
-
The understanding of redox principles is foundational for electrochemical cells.
Examples & Applications
Zinc in copper nitrate solution shows displacement leading to copper deposition.
The lack of observable reaction between copper and zinc sulfate solutions illustrates the principle of equilibrium.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Oxidation makes you lose, Reduction lets you choose - electrons gained or lost, that's the reds and oks.
Stories
Imagine a competition where metals race to grab electrons. Zinc's quick and takes the lead, leaving copper behind, demonstrating redox in action!
Memory Tools
Remember OIL RIG: Oxidation Is Loss (of electrons), Reduction Is Gain (of electrons).
Acronyms
Use REDOX to recall
Reduction is Electrons Donated
Oxidation is X-out (lost).
Flash Cards
Glossary
- Redox Reaction
A chemical reaction involving the transfer of electrons where oxidation and reduction occur simultaneously.
- Oxidation
The process of losing electrons in a chemical reaction.
- Reduction
The process of gaining electrons in a chemical reaction.
- Metal Activity Series
A list that ranks metals based on their ability to displace other metals from their compounds or solutions.
Reference links
Supplementary resources to enhance your learning experience.
