REDOX REACTIONS IN TERMS OF ELECTRON TRANSFER REACTIONS
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Redox Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Good morning, everyone! Today, we will delve into redox reactions as electron transfer reactions. Can anyone recall what we mean by 'oxidation' and 'reduction'?

I remember oxidation is when something gains oxygen, and reduction is when it loses oxygen.

Good start! However, oxidation is more broadly defined as the loss of electrons and reduction as the gain of electrons. To help you remember, think of the acronym 'LEO goes GER' - 'Lose Electrons, Oxidation' and 'Gain Electrons, Reduction'.

So, does that mean when sodium reacts with chlorine, sodium is oxidized?

Yes, exactly! Sodium donates its electrons to chlorine, which is reduced. This dual action is what characterizes redox reactions.

What happens with zinc and copper in the reactions?

Great question! Zinc loses electrons and gets oxidized while copper ions gain electrons and are reduced. Let's remember this through practical experiments.
Half Reactions in Redox
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s dissect our redox reactions into half-reactions for clarity. What do you think a half-reaction would involve?

It sounds like it’s breaking the reaction down into parts, one for oxidation and one for reduction?

Right! For instance, in the formation of sodium chloride, we can write: 2Na → 2Na⁺ + 2e⁻ for oxidation, and Cl₂ + 2e⁻ → 2Cl⁻ for reduction. Learning to express reactions this way helps us balance them.

So, if we balance the electrons lost and gained, that ensures the reactions are correct?

Exactly! It's crucial for the reactions to remain balanced. Can anyone think of how we might apply these concepts to real-life situations?

Like in batteries, where electron transfer produces electricity?

Exactly! Understanding redox reactions is vital for grasping how batteries work.
Practical Demonstration of Redox Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s explore a practical setup now! I have a zinc strip and a copper nitrate solution. What do you think will happen when we combine them?

I think the zinc will get coated in copper, since zinc is more reactive.

Exactly! Zinc gets oxidized while copper ions get reduced, which we can visualize. Do you notice any changes in the solution?

The solution turns lighter as copper gets deposited!

That's right! The blue color of copper ions decreases as they are reduced to solid copper. What does this imply about the energy transfer?

It’s like a battery, generating energy as reactions occur!

Exactly! Redox reactions drive many energy-producing processes.
Competitive Electron Transfer
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, we explore competitive electron transfer. When zinc is placed in copper sulfate, what do you think happens?

Zinc displaces copper, right? Since zinc is more reactive?

Correct, zinc displaces copper ions! This leads us to an interesting topic: metal activity series. Why do you think this hierarchy is important?

It helps predict which metals can replace others in reactions!

Exactly! Now, on the contrary, if copper were placed in a zinc sulfate solution, would anything happen?

No, because copper is less reactive than zinc.

Well done! This reactivity series is crucial in metal extraction and galvanic cell function.
Application of Redox in Cells
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let's discuss the application of these concepts in galvanic cells. Who remembers how we connect the electrodes?

With a salt bridge, to maintain the flow of charge!

Correct! A salt bridge allows ions to carry the current without mixing solutions. Can someone summarize the flow of electrons?

Electrons flow from anode to cathode through the wire, generating electricity!

Well done! This is the whole principle behind batteries powering our devices every day.

As we wrap up, let’s revisit what we learned: redox reactions involve electron transfer that is fundamental to both chemical processes and energy productions in systems like batteries.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, the concept of redox reactions is elaborated through the perspective of electron transfer, defining terms like oxidation, reduction, oxidizing agent, and reducing agent. It reinforces the importance of electron transfer in chemical reactions and discusses various experiment setups, such as the Daniell cell, to illustrate these principles.
Detailed
REDOX REACTIONS IN TERMS OF ELECTRON TRANSFER REACTIONS
This section delves into redox reactions as electron transfer processes, providing a framework to understand oxidation and reduction beyond the classical definitions. Initially, it highlights the transformations of sodium in reactions with chlorine, oxygen, and sulfur, illustrating how sodium acts as a reducing agent by losing electrons while chlorine, oxygen, and sulfur act as oxidizing agents by gaining these electrons.
Using half-reaction methods, the formulation of full reactions can be organized into distinct oxidation and reduction components. For instance, the formation of sodium chloride can be dissected into the loss and gain of electrons related to sodium and chlorine respectively. This understanding fosters a clearer visualization of how oxidation means loss of electrons and reduction implies gain ([7.2.1]).
The next part focuses on practical evidence of redox reactions through simple experiments, like the coating of zinc strips in copper(II) nitrate solutions ([7.15]), reinforcing that when one element is oxidized (loses electrons), another is simultaneously reduced (gains electrons).
Moreover, discussions on competitive electron transfer demonstrate the hierarchy of metal reactivity, emphasizing the significance of organizing metals based on their electron-releasing tendencies in contexts such as galvanic cells, wherein reactions generate electrical energy, pivotal for various industrial applications.
In summary, this section emphasizes that understanding redox reactions via electron transfer not only clarifies the fundamental principles behind chemical reactivity but also enhances practical applications across scientific domains.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Redox Reactions
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
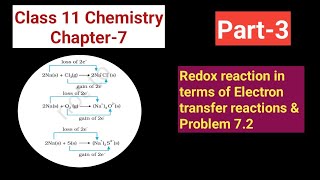
We have already learnt that the reactions
2Na(s) + Cl2(g) → 2NaCl (s) (7.12)
4Na(s) + O2(g) → 2Na2O(s) (7.13)
2Na(s) + S(s) → Na2S(s) (7.14)
are redox reactions because in each of these reactions sodium is oxidised due to the addition of either oxygen or more electronegative element to sodium. Simultaneously, chlorine, oxygen and sulphur are reduced because to each of these, the electropositive element sodium has been added.
Detailed Explanation
In redox reactions, we look at how electrons are transferred between substances. Here, sodium acts as a reducing agent by donating electrons, which oxidises sodium as it loses electrons to react with more electronegative elements like chlorine, oxygen, or sulfur. In each reaction, sodium's oxidation increases (it loses electrons), while the electronegative elements gain these electrons and are reduced.
Examples & Analogies
Think of sodium as a generous person in a group who gives away their toys (electrons) to friends (chlorine, oxygen, and sulfur) who are in need (more electronegative). By giving away these toys, sodium becomes less happy (more oxidised), while its friends become happy as they receive new toys (are reduced).
Half Reactions
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For convenience, each of the above processes can be considered as two separate steps, one involving the loss of electrons and the other the gain of electrons. As an illustration, we may further elaborate one of these, say, the formation of sodium chloride.
2 Na(s) → 2 Na+(g) + 2e–
Cl2(g) + 2e– → 2 Cl–(g)
Detailed Explanation
In redox reactions, we often break down the overall process into half-reactions. The oxidation half-reaction shows sodium losing electrons and becoming positively charged (Na⁺), while the reduction half-reaction shows chlorine gaining those electrons and becoming negatively charged (Cl⁻). This breakdown helps understand how electrons are transferred without mixing up the oxidation and reduction parts.
Examples & Analogies
Consider this a relay race where one runner (sodium) passes a baton (electrons) to another runner (chlorine). The first runner loses speed and takes on a new identity (Na⁺), while the second runner gains speed and changes into a winner (Cl⁻). Each runner's change reflects their role in the overall race (reaction).
Oxidation and Reduction Defined
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
To summarise, we may mention that
Oxidation : Loss of electron(s) by any species.
Reduction : Gain of electron(s) by any species.
Oxidising agent : Acceptor of electron(s).
Reducing agent : Donor of electron(s).
Detailed Explanation
In simple terms, oxidation is when a substance loses electrons, and reduction is when a substance gains electrons. The agent that helps in oxidation is known as the oxidising agent, and the one that helps in reduction is known as the reducing agent. This creates a system where oxidation and reduction occur simultaneously, characterizing redox reactions.
Examples & Analogies
Imagine a bank where oxidation happens when you withdraw money (electrons) from your account, reducing your balance (being oxidised). Conversely, when someone deposits money (electrons) into your account, your balance increases (being reduced). The bank manager controlling deposits is the reducing agent, and the one allowing withdrawals is the oxidising agent.
Example of Competitive Electron Transfer Reactions
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Place a strip of metallic zinc in an aqueous solution of copper nitrate as shown in Fig. 7.1, for about one hour. You may notice that the strip becomes coated with reddish metallic copper and the blue colour of the solution disappears. Formation of Zn2+ ions among the products can easily be judged when the blue colour of the solution due to Cu2+ has disappeared. The reaction between metallic zinc and the aqueous solution of copper nitrate is :
Zn(s) + Cu2+ (aq) → Zn2+ (aq) + Cu(s) (7.15)
Detailed Explanation
In this competitive electron transfer reaction, zinc gives away its electrons to copper ions, causing zinc to oxidise from Zn to Zn²⁺. Meanwhile, copper ions gain those electrons and are reduced to form solid copper. The color change in the solution (blue to colorless) indicates the consumption of copper ions as they are deposited as metallic copper on the zinc strip.
Examples & Analogies
Think of it like a cooking competition where zinc is like a chef who gives away their recipes (electrons) to copper, which is a hungry competitor. As a result, zinc runs out of recipes (turns into Zn²⁺) while copper becomes more successful by turning those recipes into delicious dishes (copper metal). The blue color of the dish disappears when all recipes are gone!
Equilibrium in Electron Transfer Reactions
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
At this stage we may investigate the state of equilibrium for the reaction represented by equation (7.15). For this purpose, let us place a strip of metallic copper in a zinc sulphate solution. No visible reaction is noticed and attempt to detect the presence of Cu2+ ions by passing H2S gas through the solution to produce the black colour of cupric sulphide, CuS, does not succeed.
Detailed Explanation
When metallic copper is placed in zinc sulfate solution, no reaction occurs because copper cannot displace zinc from the solution. Here, we can infer that zinc has a stronger tendency to lose electrons compared to copper, demonstrating the idea of electron transfer reactions being in favor of more reactive metals.
Examples & Analogies
Imagine zinc as a dominant player in a sports game where it easily scores (loses electrons) against a slower team (copper). In contrast, when the slower team (copper) plays against an equally skilled or faster rival (zinc sulfate), no points are scored (no reaction), showing who the stronger player on the field is.
Concept of Activity Series
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The competition for electrons between various metals helps us to design a class of cells, named as Galvanic cells in which the chemical reactions become the source of electrical energy.
Detailed Explanation
The activity series ranks metals based on their tendency to oxidize (lose electrons). The higher a metal is on this list, the more easily it loses electrons and the stronger its role as a reducing agent. This concept is crucial in electrochemistry, where these reactions help create galvanic cells, which produce electricity from spontaneous redox reactions.
Examples & Analogies
Think of the activity series as a popularity contest where metals are competing to be the favorite. Zinc, being the most active, gets the most votes as it easily gives away electrons, while metals like gold and silver may not be as active or popular when it comes to donating electrons.
Key Concepts
-
Redox Reactions: Involves the simultaneous occurrence of oxidation and reduction.
-
Electron Transfer: Central concept where electrons are transferred from one species to another.
-
Oxidizing and Reducing Agents: Agents that facilitate the oxidation and reduction processes.
-
Half-Reactions: The division of redox reactions into oxidation and reduction events for simplification.
-
Galvanic Cells: Devices that convert chemical energy into electrical energy by means of redox reactions.
Examples & Applications
The reaction between sodium and chlorine where sodium is oxidized and chlorine is reduced.
The displacement reaction between zinc and copper sulfate where zinc is oxidized to zinc ions and copper ions are reduced to copper metal.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Oxidation is when you lose, reduction is gain, remember these quick rules, and you'll never complain!
Stories
Imagine Sodium and Chlorine at a dance where Sodium donates an electron, lighting up Chlorine's night.
Memory Tools
LEO the lion says GER: Lose Electrons is Oxidation; Gain Electrons is Reduction.
Acronyms
REDOX
Reactions involving Electron Donor and Electron eXchanger.
Flash Cards
Glossary
- Redox Reaction
A type of chemical reaction that involves the transfer of electrons between two species, resulting in oxidation and reduction.
- Oxidation
A process that involves the loss of electrons by a substance.
- Reduction
A process that involves the gain of electrons by a substance.
- Oxidizing Agent
A substance that accepts electrons and becomes reduced in a redox reaction.
- Reducing Agent
A substance that donates electrons and becomes oxidized in a redox reaction.
- Halfreaction
The representation of either the oxidation or reduction process in a redox reaction.
- Galvanic Cell
An electrochemical cell that derives electrical energy from spontaneous redox reactions.
Reference links
Supplementary resources to enhance your learning experience.
