Kinetic Interpretation of Temperature
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Connection of Pressure and Kinetic Energy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will discuss how the kinetic energy of gas molecules directly influences pressure. Can anyone tell me what pressure is?

Pressure is the force exerted by gas molecules when they collide with the walls of their container.

Exactly! Now, if we look at the kinetic energy of these molecules, we can describe pressure mathematically. The equation \( P = \frac{n m \langle v^2 \rangle}{3} \) shows how pressure depends on the number density of molecules and their average kinetic energy.

Does this mean that if the average kinetic energy increases, the pressure also increases?

Correct! Remember this relationship; think of it as 'Pressure is like a guardian of motion,' representing the energy of molecular activity.

So, in a hot gas, there are more energetic molecules hitting the walls faster?

"You got it! This is why temperature is an indicator of the average kinetic energy of gas molecules. Let's summarize:
Kinetic Interpretation of Temperature
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let’s delve into how temperature relates to kinetic energy. Can anyone tell me what temperature measures in gases?

Isn't it a measure of how hot or cold something is?

Yes, but specifically, in the realm of kinetic theory, temperature is a measure of the average kinetic energy of molecules. The equation \( E = \frac{3}{2} k_B T \) relates these concepts.

So, as we increase the temperature, more energy means molecules move faster?

Absolutely! This can be memorized as 'Temperature numerically shares energy with kinetic motion.' Higher temperature represents higher kinetic energy, even if the gas type changes.

What about mixtures of gases? How does this work?

"Great question! In a mixture, the average kinetic energy across different gas types equates at the same temperature, showing that bonded gas mixtures behave uniformly under thermodynamic principles. Let's summarize:
Average Kinetic Energy and Root Mean Square Speed
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's explore average kinetic energy and root mean square speed. How many of you remember the equation we just discussed?

It's \( E = \frac{3}{2} k_B T \).

Perfect! Now, this leads us to another important concept: the root mean square speed, represented as \( v_{rms} \). What’s the relationship between speed and energy?

Is it that higher speeds lead to higher energy?

Exactly! The root mean square speed captures the average speed of particles, integrating their energy behavior. It's crucial in illustrating the behavior of gases under varying temperatures and conditions.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, the relationship between temperature and molecular motion is explored through kinetic theory. It articulates how the average kinetic energy of a gas is directly proportional to its absolute temperature, providing a molecular perspective on temperature and internal energy.
Detailed
Kinetic Interpretation of Temperature
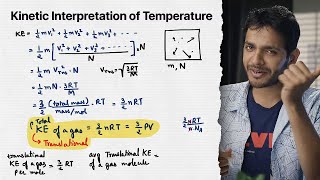
The kinetic theory of gases elucidates the relationship between temperature and the average kinetic energy of gas molecules. The pressure of an ideal gas can be expressed in terms of the kinetic energy:
- The equation \( PV = (1/3) nV m \langle v^2 \rangle \) connects pressure, volume, and molecular motion. Here, \( \langle v^2 \rangle \) is the mean square velocity of the molecules.
- The internal energy \( E \) of an ideal gas, calculated as \( E = N \times (1/2) m \langle v^2 \rangle \), highlights that for an ideal gas, the internal energy depends solely on temperature. The relationship established by \( PV = (2/3) E \) leads to the conclusion that the average kinetic energy of a molecule is proportional to the absolute temperature of the gas:
\[ E = \frac{3}{2} k_B NT \]
This equation indicates that the energy per molecule is given by \( \frac{E}{N} = \frac{3}{2} k_B T \), indicating that higher temperatures correlate with higher average kinetic energies of gas molecules.
The section also discusses mixtures of non-reactive ideal gases, concluding that at equilibrium, the average kinetic energy of the molecules in the mixture is equal regardless of their types. This lays the foundation for understanding the root mean square speed (\( v_{rms} \)), which provides another lens to view molecular velocities within gases.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Relationship Between Pressure and Energy
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Equation can be written as
PV = (1/3) nV m²v
PV = (2/3) N x ½ m²v
where N (= nV) is the number of molecules in the sample.
Detailed Explanation
The kinetic theory of gases connects physical properties such as pressure (P) and volume (V) with the average kinetic energy of gas molecules. We can express the ideal gas behavior in terms of pressure: for a given volume of gas containing a large number of molecules (N), there is a relationship that links average molecular speeds (v) to pressure and volume. The equations state that pressure is directly related to kinetic energy, showing that when molecules collide with the walls of a container, they exert a force which is perceived as pressure.
Examples & Analogies
Imagine a room filled with balloons. Each balloon represents a molecule of gas. When they bump against the walls, they push against them and make a noise, which is similar to how gas molecules push against the walls of their container. The more balloons there are and the faster they move, the louder the noise, just like increased pressure in a gas means more molecular collisions with the container walls.
Internal Energy of an Ideal Gas
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Since the internal energy E of an ideal gas is purely kinetic *, E = N × (1/2) m²v
Equation then gives :
PV = (2/3) E
Detailed Explanation
In the context of an ideal gas, all internal energy is kinetic. As the gas molecules move, their motion contributes to the internal energy of the gas. The total internal energy can be expressed as a function of the number of molecules (N) and the average kinetic energy of each molecule. This connection between pressure, volume, and internal energy can be established through physical laws governing energy conservation in elastic collisions.
Examples & Analogies
Think of a group of children running around in a large playground. The energy they have from running is similar to the internal energy in gas molecules. When the children collide with the fence (the walls of the playground), they exert a force; similarly, gas molecules exert pressure when they collide with the walls of their container. If all the children were to stop running, there would be no energetic 'bounces' against the walls.
Temperature and Kinetic Energy Relationship
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
We are now ready for a kinetic interpretation of temperature. Combining with the ideal gas , we get E = (3/2) kB NT
or E/N = (1/2) m²v = (3/2) kBT
Detailed Explanation
This relationship shows that the average kinetic energy (E/N) of a molecule in an ideal gas is directly proportional to the absolute temperature (T). This means that as the temperature of the gas increases, the average speed of the molecules increases, leading to greater kinetic energy. This establishes temperature as a measure of how much energy the gas molecules possess as they move.
Examples & Analogies
Imagine heating a pot of water on a stove. As the water heats up (increased temperature), the molecules in the water start moving faster and faster, which is why you see bubbles forming and steam rising when it reaches boiling point. The heat that's added to the pot directly corresponds to the increase in temperature and the kinetic energy of the water molecules.
Implications for Mixtures of Gases
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For a mixture of non-reactive ideal gases, the total pressure gets contribution from each gas in the mixture. Equation becomes P = (1/3) [ n1m₁²v₁ + n2m₂²v₂ +…]
Detailed Explanation
When you have a mixture of different gases, each gas contributes to the total pressure based on its number density, molecular mass, and kinetic energy. The formula shows how these contributions are calculated, indicating that the total pressure is the sum of pressures contributed by each gas. This leads to Dalton’s Law of Partial Pressures, where pressure is proportional to the individual components of the gas mixture.
Examples & Analogies
Consider a fruit salad with different types of fruits. Each type of fruit adds its unique flavor to the salad, just like each gas adds its individual pressure to the total pressure of a gas mixture. If there is more of one fruit (gas) than another, it will dominate the flavor (pressure) of the salad.
Speed of Gas Molecules at Different Temperatures
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
At a temperature T = 300 K, the mean square speed of a molecule in nitrogen gas is:
2v = (3 kB T) / m = (516)² m²s⁻²
The square root of 2v is known as root mean square (rms) speed and is denoted by vrms, ( We can also write 2v as < v² >.)
vrms = 516 m s⁻¹
Detailed Explanation
The mean square speed of gas molecules indicates their average kinetic motion, which can be calculated using Boltzmann's constant and the mass of the gas molecule. This speed helps predict how fast the molecules are moving at a specific temperature, showing that lighter gases tend to move faster than heavier gases at the same temperature.
Examples & Analogies
Think of runners in a race. The lighter runners (like helium molecules) generally have an easier time moving faster than heavier runners (like sulfur hexafluoride molecules) when all are running on the same track (under the same temperature conditions). This can help illustrate how molecular weight influences speed in gases.
Key Concepts
-
Temperature: A measure of the average kinetic energy of gas molecules.
-
Pressure: The force exerted by gas molecules upon colliding with the walls of their container.
-
Kinetic Energy of Gas: Proportional to the temperature; higher temperatures lead to higher kinetic energy.
-
Internal Energy: Dependent solely on temperature for an ideal gas and related to pressure and volume.
-
Root Mean Square Speed: A measure of the average speed of gas molecules, linked to temperature.
Examples & Applications
Example of determining gas pressure based on kinetic energy and molecular speed.
Illustration of how temperature affects the speed of gas molecules in everyday scenarios.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Pressure and temperature rise, as energy within molecules flies.
Stories
Imagine a party where all gas molecules are dancing; the faster they dance, the higher the temperature. The pressure builds as they collide against the walls, reflecting their joyous motion.
Memory Tools
P=KE; remember as 'Pressure equals Kinetic Energy'.
Acronyms
TKP
Temperature (T)
Kinetic Energy (K)
Pressure (P).
Flash Cards
Glossary
- Kinetic Energy
The energy that a body possesses due to its motion, proportional to the mass and square of the speed.
- Absolute Temperature
A measure of temperature used in some scientific contexts, typically in Kelvin, where absolute zero is 0 K.
- Root Mean Square Speed (vrms)
A statistical measure of the speed of particles in a gas, representative of the average kinetic energy.
- Internal Energy (E)
The total energy contained within a system, reflecting the kinetic energy of its constituent molecules.
- Avogadro's Law
A principle stating that equal volumes of gases at the same temperature and pressure contain an equal number of molecules.
Reference links
Supplementary resources to enhance your learning experience.
