LAW OF EQUIPARTITION OF ENERGY
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding the Basics of the Law of Equipartition
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will discuss the Law of Equipartition of Energy, which describes how energy is distributed among the various degrees of freedom of molecules in a gas. Can anyone tell me what we mean by degrees of freedom?

Is it the various ways in which a molecule can move?

Exactly! Now, for each degree of freedom, the average energy is \(\frac{1}{2} k_B T\). Can anyone remind us what \(k_B\) stands for?

It's Boltzmann's constant!

Right! So, if we consider a monoatomic gas, how many degrees of freedom does it have, and what is the resulting average energy per molecule?

It has three translational degrees of freedom, leading to an average energy of \(\frac{3}{2} k_B T\)!

Well done! Thus, for monoatomic gases, each degree of freedom contributes \(\frac{1}{2} k_B T\), summing up to \(\frac{3}{2} k_B T\). Remember this format as we go deeper!
Applying the Law to Diatomic and Polyatomic Gases
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let\u2019s consider a diatomic gas, like O2. How does the number of degrees of freedom change?

It has three translational and two rotational degrees of freedom, making it five in total!

Correct! This gives the diatomic gas a total energy of \(\frac{5}{2} k_B T\). Now what about if we consider vibrational modes?

Vibrational modes would add more energy because they have both kinetic and potential components.

Exactly! Each vibrational frequency contributes \(k_B T\), doubling the contribution of each vibrational mode. Now, can anyone summarize how energy is expressed for these molecules?

For diatomic, it's \( U = \frac{5}{2} k_B T + v k_B T\) where 'v' is the number of vibrational modes.

Perfect! This gives us a comprehensive view of how the energy is divided in various gas molecules.
Understanding Specific Heats in Context
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let\u2019s talk about specific heats. How do you think this law connects to the specific heats at constant volume and pressure?

I think it will show us how the amount of energy contributes to temperature change measurements!

That's right! For a monatomic gas, the molar specific heat at constant volume \(C_v\) is calculated as \(C_v = \frac{3}{2} R\), where \(R\) is the ideal gas constant. Can anyone explain for diatomic gases?

For diatomic gases, \(C_v = \frac{5}{2} R\).

Correct! And for polyatomic gases? What do you think is the formula?

It will include rotational and vibrational contributions as well, so it would be \(C_v = (3 + f) R\), where 'f' represents vibrational degrees.

Exactly! This knowledge helps understand how gases behave and the role of temperature in their energy states!
Recap and Real-World Applications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

So, to recap, what have we learned about the Law of Equipartition of Energy?

We learned that energy is distributed equally across different degrees of freedom at thermal equilibrium.

And that for each type of molecule \u2014 mono, di, and polyatomic \u2014 the energy contributions change!

Exactly! Now, why is this important? How does it apply to things like engines or refrigeration?

Understanding these principles helps us design better thermal systems by knowing how energy is managed.

Spot on! Equipartition leads into many applications in real-world physics, especially pertaining to heat engines and specific heat calculations.

It really connects physics to practical scenarios!

Yes! Always remember, a topic's equation relates to its real-world application.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
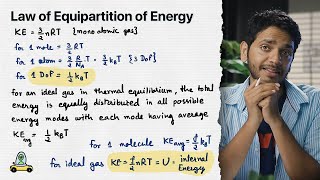
In this section, the Law of Equipartition of Energy is discussed in detail, explaining how energy divides into various modes, including translational, rotational, and vibrational, and how this affects the average energy and specific heats of gases. It highlights the significance of degrees of freedom in the context of different types of molecules.
Detailed
Detailed Summary\n\nThe Law of Equipartition of Energy explains how energy is distributed in a system at thermal equilibrium. This law states that for each degree of freedom available to a gas molecule, the average energy is equal to \n\n$$\frac{1}{2} k_B T$$\n\nwhere:\n- $k_B$ is the Boltzmann constant,\n- $T$ is the absolute temperature.\n\nIn the context of a monoatomic gas, only translational degrees of freedom exist, leading to an average energy of \n\n$$\frac{3}{2} k_B T$$\n\nfor each molecule. In contrast, diatomic gases like O2 or N2 have three translational and two rotational degrees of freedom, totaling five degrees, which results in an internal energy of \n\n$$\frac{5}{2} k_B T$$\n\nFurthermore, if a molecule is able to vibrate, it contributes additional energy with both kinetic and potential components, leading to further complexity in its energy expressions. \n\nThus, the total energy for molecules can be expressed as:\n\n$$E = \frac{3}{2} N k_B T + f k_B T$$\n\nwhere f represents the number of vibrational degrees of freedom. Moreover, vibrations contribute more because they involve both kinetic and potential energy components, equating to a contribution of $k_B T$ per vibrational mode. \n\nIn summary, this section establishes that the total energy in a system is equally distributed across all modes of energy, which underpins the thermodynamic properties of gases such as specific heat capacities.
Youtube Videos



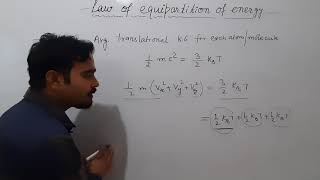




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Kinetic Energy of Molecules
Chapter 1 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The kinetic energy of a single molecule is
$$\epsilon = \frac{1}{2} mv_x^2 + \frac{1}{2} mv_y^2 + \frac{1}{2} mv_z^2$$ (12.22)
Detailed Explanation
The kinetic energy ($\epsilon$) of a molecule is given by the formula where $m$ is the mass of the molecule and $v_x$, $v_y$, and $v_z$ are the velocities of the molecule in the x, y, and z directions, respectively. This equation indicates how energy is distributed in a molecule's movement across three dimensions.
Examples & Analogies
Imagine a basketball moving in a three-dimensional space. The way the ball moves forward, sideways, and upwards corresponds to its different velocity components (x, y, z). The faster it rolls in those directions, the more kinetic energy it has.
Average Energy in Thermal Equilibrium
Chapter 2 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For a gas in thermal equilibrium at temperature T, the average value of energy denoted by ⟨ε⟩ is
$$\langle\epsilon\rangle = \frac{3}{2} k_B T$$ (12.23)
Detailed Explanation
In thermal equilibrium, the average kinetic energy ($\langle\epsilon\rangle$) of molecules in a gas is directly proportional to the temperature ($T$) multiplied by Boltzmann's constant ($k_B$). This relationship shows that as temperature increases, the average kinetic energy of the gas molecules also increases, thus reflecting more vigorous motion.
Examples & Analogies
Think about how the temperature of a pot of water increases as you heat it. The greater the heat (or temperature), the more vigorously the water molecules move around, demonstrating the direct relationship between temperature and kinetic energy.
Degrees of Freedom
Chapter 3 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A molecule free to move in space needs three coordinates to specify its location. This can also be expressed in another way. We say that it has one degree of freedom for motion in a line, two for motion in a plane, and three for motion in space (12.24)
Detailed Explanation
Degrees of freedom refer to the number of independent ways in which a system can move. For a molecule in three-dimensional space, it has three degrees of freedom corresponding to its ability to move in three perpendicular directions (x, y, z). This concept helps in understanding how the energy is distributed in a gas.
Examples & Analogies
Consider a bird flying in an open sky. It can move forward and backward (along the x-axis), up and down (along the y-axis), and turn around (along the z-axis). Each of these movements represents a degree of freedom.
Translational and Rotational Degrees of Freedom
Chapter 4 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Molecules of a monatomic gas like argon have only translational degrees of freedom. But what about a diatomic gas such as O2 or N2? A molecule of O2 has three translational degrees of freedom and can also rotate about its center of mass (12.25)
Detailed Explanation
Monatomic gases possess only translational degrees of freedom, meaning they can only move in space. Diatomic gases, such as oxygen (O2), have both translational and rotational degrees of freedom, allowing them to move as well as rotate. This leads to different contributions to the energy of the molecule.
Examples & Analogies
Visualize a single solid ball representing a monatomic gas which can only roll along the ground versus a toy model of a figure-eight roller coaster representing diatomic gas that can roll and also twist or turn in different directions. The complexity of motion increases with more degrees of freedom.
Vibrational Energy Mode
Chapter 5 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
One vibrational mode contributes two 'squared terms': kinetic and potential energies (12.26)
Detailed Explanation
In addition to translational and rotational modes, some molecules can vibrate, creating a vibrational energy mode. For each vibrational mode, since there are motions in both kinetic and potential forms, it contributes more to the overall energy, effectively making it higher than other modes.
Examples & Analogies
Think of a guitar string. As you pluck it, the string vibrates back and forth (kinetic energy), and also gets pulled taut and relaxed (potential energy). The energy involved in both the stretching and moving of the string reflects the dual contribution of vibrational energy modes.
Law of Equipartition of Energy
Chapter 6 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In equilibrium, the total energy is equally distributed in all possible energy modes, with each mode having an average energy equal to ½ k_BT. This is known as the law of equipartition of energy (12.26)
Detailed Explanation
The law of equipartition of energy states that in thermal equilibrium, energy is distributed equally among all available degrees of freedom of a system. Each degree of freedom contributes an energy of ½ k_B T, which reflects energy conservation and distribution principles in statistical mechanics.
Examples & Analogies
Imagine a classroom during a group project. If each group member is contributing equally to the task (equilibrium), everyone’s contribution would be similar. If one person works much harder, others would need to catch up to balance the contributions just as energy balances out in physical systems.
Energy Contributions from Different Modes
Chapter 7 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Accordingly, each translational and rotational degree of freedom contributes ½ k_B T to the energy, while each vibrational frequency contributes k_B T (12.26)
Detailed Explanation
Each degree of freedom for translation and rotation contributes an energy amount of ½ k_B T, while each vibrational degree contributes k_B T due to both kinetic and potential contributions. This helps in calculating specific heats for different types of gases based on their molecular structure.
Examples & Analogies
Think of a cooking pot on a stove. The heat adds energy, causing the water to move (kinetic energy) and vibrate (potential energy). If you think of each movement type as contributing differently to cooking, it mirrors how different motions contribute to the energy of molecules.
Key Concepts
-
Equipartition of Energy: Energy is distributed equally among all degrees of freedom.
-
Degrees of Freedom: Refers to independent ways a molecule can move or rotate.
-
Translational, Rotational, and Vibrational Energies: Types of energy corresponding to movement, rotation, and vibration of molecules, respectively.
-
Specific Heat Capacity: The heat required to change the temperature of one mole of a substance by one degree Celsius.
Examples & Applications
Monatomic gases have three translational degrees of freedom, contributing \(\frac{3}{2} k_B T\) to their average energy.
Diatomic gases like O2 have five degrees of freedom (three translational and two rotational) leading to an average energy of \(\frac{5}{2} k_B T\).
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Equipartition ensures fair play, energy's spread is here to stay.
Stories
Imagine a party with different dance floors, each groove allows movements; energy must flow evenly throughout the floor!
Memory Tools
D for Degrees, E for Energy; Equipartition guides them like an energy symphony.
Acronyms
E = 1/2kBT
Remember Equipartition - Energy is half times kB times T!
Flash Cards
Glossary
- Law of Equipartition of Energy
A principle stating that energy is equally distributed among all degrees of freedom in a system at thermal equilibrium.
- Degrees of Freedom
The number of independent ways in which a system can possess energy; each degree contributes a set amount of energy in thermal equilibrium.
- Translational Energy
Energy associated with the motion of a molecule moving from one location to another.
- Rotational Energy
Energy associated with the rotation of a molecule around its center of mass.
- Vibrational Energy
Energy associated with the vibration of atoms within a molecule, involving both kinetic and potential energy.
- Molar Specific Heat
The amount of heat required to raise the temperature of one mole of a substance by one degree Celsius.
- Boltzmann Constant (k_B)
A physical constant relating the average kinetic energy of particles in a gas with the temperature of the gas.
- Ideal Gas Constant (R)
The constant used in the ideal gas law representing the relationship between pressure, volume, temperature, and amount of substance.
Reference links
Supplementary resources to enhance your learning experience.
