Factors Influencing Rate of Reaction
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Influence of Concentration on Rate
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's start our discussion by exploring how the concentration of reactants can influence the rate of a reaction. When we increase the concentration, what do you think happens?

I think the rate of the reaction would increase because there are more particles to collide.

That's correct! This relationship can be expressed in the rate law. We say the rate is proportional to the concentrations raised to some power. Can anyone tell me why higher concentrations lead to more collisions?

It’s because there are more molecules in a given volume, right?

Exactly! So, remember, for a reaction of the form aA + bB → cC + dD, we can express this as Rate = k[A]^x[B]^y. The x and y are determined experimentally.

Does this mean different reactions can have different values for x and y?

Yes! They can be equal to or different from the stoichiometric coefficients. This is why the rate law must be determined through experimentation. Let's summarize: concentration increases due to more collisions, and this can be quantitatively expressed in the rate law.
Temperature's Role
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let’s discuss temperature. How does an increase in temperature generally affect reaction rates?

I think it speeds up the reaction because the molecules move faster.

Exactly right! Higher temperatures increase the kinetic energy of molecules, leading to more frequent and more energetic collisions. This can be represented by the Arrhenius equation. Can anyone explain what activation energy is?

It's the minimum energy required for a reaction to occur, right?

Correct! So when we increase temperature, we not only increase the frequency of collisions, but also the number of particles that have enough energy to overcome this activation energy barrier.

Does that mean even small increases in temperature can have a big impact on the rate?

Precisely! When the temperature increases by about 10 degrees Celsius, the rate constant often doubles for many reactions. Let’s summarize this idea: temperature increases kinetic energy, boosting both collision frequency and energy.
Catalysts and Their Function
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s move on to catalysts. Who can tell me what a catalyst does?

It speeds up a reaction without being consumed in the process.

That's correct! Catalysts work by providing an alternative reaction pathway with a lower activation energy. Can anyone think of a real-world example of a catalyst?

In cars, catalytic converters speed up reactions that reduce harmful emissions.

Excellent example! Remember that while catalysts speed up both the forward and reverse reactions equally, they do not affect the equilibrium position. Let’s summarize: a catalyst lowers activation energy and allows a reaction to occur faster without being consumed.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
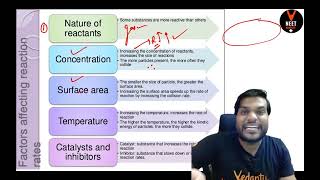
The rate of a chemical reaction is influenced by several experimental conditions, primarily the concentration of reactants, temperature, and the presence of catalysts. This relationship is captured in rate laws which help determine how changes in these factors affect chemical reaction rates.
Detailed
Factors Influencing Rate of a Reaction
Chemical kinetics encompasses the study of reaction rates and the factors affecting those rates. The rate of a chemical reaction depends significantly on experimental conditions, including:
- Concentration: The rate of a reaction often increases with the concentration of reactants because more molecules are available to collide and react. This relationship is formalized in the concept of the rate law, which defines the rate of a reaction in terms of reactant concentrations.
- Temperature: Increasing the temperature typically increases reaction rates by providing reactant molecules with more kinetic energy, thus enhancing collision frequency and energy, raising the number that can overcome activation energy barriers.
- Catalysts: The presence of a catalyst speeds up a reaction without being consumed, by reducing the activation energy. Catalysts provide an alternative reaction pathway, which results in increased reaction rates.
The mathematics of these concepts is explained through differential rate equations and integrates the impact of concentration into the rate of reaction, highlighting that factors like molecularity and order also play crucial roles. The section concludes with insights into terms such as rate constant and the order of a reaction which is experimentally determined and influenced by these factors.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Dependence of Rate on Concentration
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The rate of a chemical reaction at a given temperature may depend on the concentration of one or more reactants and products. The representation of rate of reaction in terms of concentration of the reactants is known as rate law. It is also called as rate equation or rate expression.
Detailed Explanation
The rate of a chemical reaction can change based on how concentrated the reactants and products are. A higher concentration generally means that more reactant particles are available to collide and react, thus speeding up the reaction. The relationship between the reaction rate and concentrations of reactants is expressed mathematically in the rate law, which is the equation that defines how the rate depends on these concentrations.
Examples & Analogies
Think of a crowded party where everyone wants to dance. If there are just a few people on the dance floor (low concentration), they have to wait for a turn. But if there are many dancers (high concentration), they can easily form groups and dance faster. Similarly, a higher concentration of reactants increases the chances of collisions, thus speeding up the reaction.
Rate Expression and Rate Constant
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The results clearly show that rate of a reaction decreases with the passage of time as the concentration of reactants decrease. Conversely, rates generally increase when reactant concentrations increase. So, the rate of a reaction depends upon the concentration of reactants.
Detailed Explanation
As a reaction proceeds, the amounts of reactants diminish, leading to a slower reaction rate. Conversely, if we increase the concentrations of the reactants, there will be more molecules available to collide, increasing the likelihood of reactions and thus increasing the rate. This relationship forms part of the rate expression, which includes the rate constant, a value that is specific to each reaction at a given temperature.
Examples & Analogies
Imagine you're baking cookies. If you add more flour (a reactant), you'll have more cookie dough to work with, allowing you to create cookies more quickly. This is like increasing the concentration of a reactant, which can lead to a higher reaction rate.
Order of a Reaction
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In the rate equation, the sum of the exponents, i.e., x + y, gives the overall order of a reaction whereas x and y represent the order with respect to the reactants A and B respectively. Hence, the sum of powers of the concentration of the reactants in the rate law expression is called the order of that chemical reaction.
Detailed Explanation
The order of a reaction is determined by the exponents in the rate law equation. It indicates how sensitive the reaction rate is to changes in reactant concentrations. For example, if the rate doubles when the concentration of a reactant A is doubled and is represented as first order with respect to A. The overall order of the reaction is simply the sum of all the orders with respect to each reactant.
Examples & Analogies
Consider a plant growing faster in a nutrient-rich soil (higher concentration). If you double the nutrients (first order), the growth rate might double. If the growth rate changes more significantly with more nutrients, we can describe the growth with a higher order, indicating it reacts differently to the changes in conditions.
Molecularity of a Reaction
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The number of reacting species (atoms, ions or molecules) taking part in an elementary reaction is called molecularity of a reaction. The reaction can be unimolecular when one reacting species is involved, bimolecular when two are involved, and termolecular when three interacting species collide.
Detailed Explanation
Molecularity is a concept that applies specifically to elementary reactions, which occur in a single step. For example, in a unimolecular reaction, one molecule reacts to form products. In a bimolecular reaction, two molecules collide to generate products. Though rare, a termolecular reaction involves three reacting species. The molecularity provides insights into how many particles must collide for a reaction to occur.
Examples & Analogies
Imagine a single ball bouncing off a wall (unimolecular), two players passing a ball to each other (bimolecular), or three friends forming a circle to pass a ball (termolecular). The molecularity concept illustrates how different particle interactions can define how reactions occur.
Effect of Catalyst
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A catalyst is a substance which increases the rate of a reaction without itself undergoing any permanent chemical change. It helps in attaining the equilibrium faster, catalyzing both forward and backward reactions to the same extent.
Detailed Explanation
Catalysts speed up reactions by providing an alternative pathway with a lower activation energy. They remain unchanged at the end of the reaction, allowing them to be used repeatedly. This means that the presence of a catalyst can significantly increase the speed of a reaction without being consumed in the process.
Examples & Analogies
Think of a carpool lane that allows a few cars to move faster than traffic. Here, the cars are like the catalyst; they enable a faster commute without altering the overall route. Similarly, a catalyst facilitates chemical reactions, speeding them up without being used up.
Key Concepts
-
Rate of Reaction: The change in concentration of reactants or products over time.
-
Factors Influencing Rate: Concentration, temperature, and catalysts all significantly impact reaction rates.
-
Rate Law: An equation that expresses the relationship between the rate of reaction and the concentrations of reactants.
Examples & Applications
Increasing the concentration of reactants typically results in an increased rate of reaction.
The use of a catalyst can speed up the production of reactants by lowering the activation energy required for the reaction.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In reaction's dance, when temp does rise, faster they bump, under bright skies.
Stories
Imagine a crowded room where everyone wants to mingle. As more people enter, interactions become more frequent, similar to how increased concentration leads to higher reaction rates.
Memory Tools
CAT for concentration, activation, and temperature—remember these for reaction rates!
Acronyms
CATS
Concentration
Activation energy
Temperature
and Speed—elements of reaction rates.
Flash Cards
Glossary
- Rate Law
An equation that relates the rate of a reaction to the concentrations of the reactants.
- Activation Energy
The minimum energy required for a reaction to occur.
- Catalyst
A substance that increases the rate of a reaction without being consumed.
- Order of a Reaction
The sum of the powers of the concentration terms in the rate law.
- Rate Constant
A proportionality constant in the rate law that is dependent on temperature and the presence of a catalyst.
Reference links
Supplementary resources to enhance your learning experience.
