Rate Expression and Rate Constant
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Rate Expressions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's begin by discussing rate expressions. Can anyone tell me what a rate expression is in the context of a chemical reaction?

I think it's a way to show how the rate of a reaction depends on the concentrations of reactants.

Exactly! The rate expression shows how the rate relates to the concentrations of reactants. For example, for the reaction aA + bB -> cC + dD, we can write it as Rate = k[A]^x[B]^y.

What do the 'x' and 'y' represent?

Great question! They represent the order of the reaction with respect to each reactant. The order tells us how sensitive the rate is to the concentration changes. Now, let's use a quick mnemonic to remember this: 'Rate is k times the powers of concentrations' - R = k[A]^x[B]^y.

So if we change the concentrations, we can predict how the rate will change, right?

Correct! The ability to predict reaction rates based on concentration changes is crucial in kinetics.
Understanding the Rate Constant
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we've discussed rate expressions, let’s delve into the rate constant, denoted as 'k'. Can anyone explain what 'k' represents in a rate expression?

I think it's a constant that relates the rate of a reaction to the concentrations of reactants.

Exactly! 'k' is crucial because it quantitatively connects reaction rates to the concentrations of the reactants. It varies based on temperature and sometimes the presence of a catalyst. For example, if we look at a first-order reaction, a doubling of the concentration results in doubling the rate.

What about the units of 'k'? Do they change?

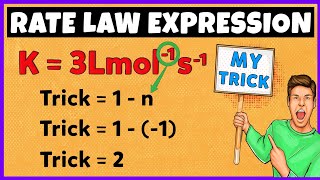
Yes, they do! The units of 'k' depend on the overall order of the reaction. For a zero-order reaction, the units are mol L⁻¹ s⁻¹, whereas for a first-order reaction, it's s⁻¹. Let's remember: 'Zero - mols over time, First - just time!'

That's a helpful way to keep track of the units!
Application of Rate Laws
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Using experimental data, we can derive specific rate laws. Let’s say we conduct an experiment and find that when the concentration of NO increases, the rate quadruples, while keeping O₂'s concentration constant. What does this say about the order with respect to NO?

That means the reaction is second order with respect to NO!

Exactly, and if we summarize it—our rate expression would look like Rate = k[NO]^2[O₂]. Remember, every reaction results in rate laws that need to be determined through experiments, not guessed from the balanced equation.

So experiments help us understand not just the how but also the why behind the rates, right?

Absolutely! Experimental determination is a key point in chemical kinetics.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, we explore the relationship between reaction rates and reactant concentrations, establishing the framework for rate expressions. The concept of the rate constant is introduced, highlighting its significance in determining the kinetics of chemical reactions. Examples illustrate how to derive and apply rate laws based on experimental data.
Detailed
Rate Expression and Rate Constant
Chemical kinetics focuses on how quickly reactions occur and the factors that influence reaction rates. A fundamental aspect of this field is the understanding of rate expressions and the rate constant, which connects the rate of a reaction to the concentrations of reactants.
Rate Expressions
Rate expressions describe how the reaction rate correlates with concentrations of reactants. For a general reaction:
$$ aA + bB \rightarrow cC + dD $$
The rate expression can be defined as:
$$ Rate \propto [A]^x [B]^y $$
Where:
- x and y are the reaction orders with respect to reactants A and B, respectively.
This can also be expressed as:
$$ Rate = k[A]^x[B]^y $$
Here, k is the rate constant, a proportionality factor that varies with temperature and specific to each reaction.
Rate Constant and Its Units
The value of the rate constant k is pivotal in kinetics. It is influenced by factors such as temperature and the presence of catalysts, with units that depend on the overall order of the reaction.
- For a zero order reaction, units of k are mol L⁻¹ s⁻¹.
- For a first order reaction, k's units are s⁻¹.
- For a second order reaction, units are mol⁻¹ L s⁻¹.
Examples of Rate Laws
Through experimentation, specific rates can be determined. For instance, if doubling the concentration of NO in the reaction below:
$$ 2NO + O_2 \rightarrow 2NO_2 $$
leads to a quadrupling of the rate, we deduce that:
$$ Rate = k[NO]^2[O_2]^1 $$
Significance in Chemical Kinetics
Understanding rate expressions and constants aids chemists in predicting how reactions behave under varying conditions, providing insights into mechanism and reactivity.
In summary, the rate of a reaction is determined by the nature of reactants, concentrations involved, and temperature, summarized mathematically in rate expressions that allow prediction and analysis of reaction dynamics.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Overview of Rate Expression
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The results in Table 3.1 clearly show that rate of a reaction decreases with the passage of time as the concentration of reactants decrease. Conversely, rates generally increase when reactant concentrations increase. So, rate of a reaction depends upon the concentration of reactants.
Detailed Explanation
This chunk explains how the rate of a reaction is affected by the concentration of reactants. As reactants are consumed, their concentrations decrease leading to a slower reaction rate. Conversely, if the concentration of reactants is increased, the reaction rate typically also increases because more reactant particles are available to collide and react.
Examples & Analogies
Think of a crowded room. If more people (reactants) enter the room, the chances of them bumping into each other (reacting) increase, speeding up the social interactions (reaction rate). Conversely, as people leave the room, interactions slow down because fewer people are left.
General Rate Expression
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Consider a general reaction aA + bB → cC + dD, where a, b, c, and d are the stoichiometric coefficients of reactants and products. The rate expression for this reaction is Rate µ [A][B], where exponents x and y may or may not be equal to the stoichiometric coefficients (a and b) of the reactants.
Detailed Explanation
In a chemical reaction, the rate at which products are formed or reactants are consumed depends on their concentrations. The rate expression shows that the reaction rate is proportional to the product of the concentrations of the reactants, each raised to a power. These powers (exponents) do not always match the stoichiometric coefficients found in the balanced chemical equation; they must be determined experimentally.
Examples & Analogies
Imagine baking cookies. If you double the amount of dough (A) and keep the same amount of sugar (B), it’s not just about doubling the dough; sometimes it changes how sweet your cookies are. Similarly, concentration changes influence reaction rates in non-linear ways.
Differential Rate Equation and Rate Constant
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The equation can also be written as Rate = k[A]x[B]y, where k is a proportionality constant called rate constant. This form of the equation is known as the differential rate equation, where rate is related to concentration of reactants and k is crucial for understanding the speed of a reaction.
Detailed Explanation
The differential rate equation represents how the rate of a reaction depends on the concentrations of its reactants. The constant k reflects how fast the reaction can proceed when reactant concentrations change. Knowing k is essential for predicting reaction behavior under different conditions.
Examples & Analogies
Think about a race. The speed at which the car (reaction) goes can depend on factors like engine power (k) and the amount of fuel (A and B). Just like how adding more fuel can potentially speed up the race, increasing reactant concentrations can enhance the reaction rate.
Rate Law and Experimental Determination
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Rate law is the expression in which reaction rate is given in terms of molar concentration of reactants with each term raised to some power, which may or may not be the same as the stoichiometric coefficient of the reacting species in a balanced chemical equation.
Detailed Explanation
Rate law quantitatively describes how the rate of a reaction depends on the concentrations of reactants. Unlike stoichiometry, which provides a theoretical basis, experimental measurements must be taken to determine the actual rate law for a reaction, including how changes in concentration affect the reaction speed.
Examples & Analogies
Imagine a chef following a recipe. The amounts of each ingredient (reactants) affect the final dish (product) differently. The chef needs to experiment to find out the right balance for optimal flavor (reaction speed), rather than just following the recipe exactly.
Key Concepts
-
Rate Expression: Represents the relationship between the reaction rate and reactant concentrations.
-
Rate Constant (k): The constant value that links reaction rate with reactant concentrations, varying with temperature and catalytic activity.
-
Order of Reaction: Defines how the rate is affected by changes in concentration, calculated from the rate law.
Examples & Applications
Through experimentation, specific rates can be determined. For instance, if doubling the concentration of NO in the reaction below:
$$ 2NO + O_2 \rightarrow 2NO_2 $$
leads to a quadrupling of the rate, we deduce that:
$$ Rate = k[NO]^2[O_2]^1 $$
Significance in Chemical Kinetics
Understanding rate expressions and constants aids chemists in predicting how reactions behave under varying conditions, providing insights into mechanism and reactivity.
In summary, the rate of a reaction is determined by the nature of reactants, concentrations involved, and temperature, summarized mathematically in rate expressions that allow prediction and analysis of reaction dynamics.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Rate increases, concentrations dance, k is the constant, give it a chance.
Stories
Imagine a chef preparing a dish; his ingredients (reactants) must be just right to cook quickly (rate). The relationship between ingredient amounts (concentrations) and cooking speed (rate) is his secret formula (rate expression)!
Memory Tools
To remember reaction orders: 'Adding powers harvests rates', emphasize to collect the powers of reactants' concentrations.
Acronyms
RAP - Rate = k[A]^x[B]^y summarizes rate expression.
Flash Cards
Glossary
- Rate Expression
A mathematical relationship that relates the rate of a reaction to the concentrations of reactants.
- Rate Constant (k)
A proportionality factor in the rate law that relates the rate of reaction to the concentrations of reactants.
- Order of Reaction
The sum of the powers of the concentrations of reactants in the rate law, indicating how the rate responds to changes in concentration.
- Differential Rate Equation
An equation that describes how the rate of a reaction depends on the concentrations of reactants.
Reference links
Supplementary resources to enhance your learning experience.
