FORMULA UNIT MASS
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Overview of Formula Unit Mass
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will learn about formula unit mass. Can anyone tell me what mass is in terms of chemistry?

Isn't mass the amount of matter in an object?

Correct! Now, in chemistry, we also refer to the mass of compounds. Who can tell me the difference between molecular mass and formula unit mass?

Molecular mass applies to molecules, and formula unit mass applies to ionic compounds?

Exactly! Formula unit mass is the sum of the atomic masses in an ionic compound. Let’s look at an example together.

What’s an example of an ionic compound?

Sodium chloride, NaCl, is a good example. Can someone calculate its formula unit mass?

I can! It would be 23 u for sodium and 35.5 u for chlorine. So, 58.5 u in total?

Perfect! Let's remember the acronym 'MASS' – M for molecular, A for atomic, S for sum, and S for simple!

To summarize, formula unit mass is crucial for understanding the composition of ionic compounds.
Calculating Formula Unit Mass
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand the concept, let’s practice calculating formula unit mass. Who wants to try calcium chloride, CaCl2?

How do we start?

First, we need the atomic masses. Calcium is 40 u, and chlorine is 35.5 u. Remember to account for two chlorines in the formula.

Right! So, it would be 40 + (2 × 35.5)?

Exactly! Can you calculate that?

That would equal 40 + 71, which is 111 u!

Great job! Remember, the formula unit mass helps us understand ionic compounds better. Let’s do one more!

What about magnesium oxide, MgO?

Perfect! What are the atomic masses?

Magnesium is 24 u and oxygen is 16 u.

Correct! Now add them up for the formula unit mass.

So, 24 + 16 = 40 u!

Well done! Always remember, practice makes perfect!
Significance of Formula Unit Mass
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s talk about why knowing formula unit mass is important. Can anyone think of a reason?

It must help in balancing chemical equations, right?

Exactly! Understanding the formula unit mass allows chemists to predict how much of each reactant is needed. Let’s see how we can apply this.

What if we have sodium sulfate?

Good question! Sodium sulfate, Na2SO4, has how many sodiums?

Two sodiums. So, we need to consider their masses.

Correct! The atomic mass of sodium is about 23 u. Let’s calculate formula unit mass.

So, it would be 2 × 23 + 32 + 4 × 16 = 142 u!

Excellent work! Now you all understand how formula unit mass ties into real-world chemistry.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Formula unit mass is defined as the sum of the atomic masses of all atoms in a formula unit of a compound. This section elaborates on the process of calculating formula unit mass, specifically for ionic compounds, and compares it with molecular mass.
Detailed
Formula Unit Mass
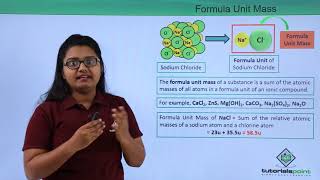
In chemistry, the concept of formula unit mass refers to the sum of the atomic masses of all atoms in a formula unit of a compound. While molecular mass applies to molecular compounds where constituent particles are molecules, formula unit mass is specifically used for ionic compounds and is calculated in much the same way. For instance, in sodium chloride (NaCl), the formula unit mass can be derived as follows:
- Identify the atomic masses:
- Sodium (Na) = 23 u
- Chlorine (Cl) = 35.5 u
- Calculate the formula unit mass:
- Formula Unit Mass of NaCl = 1 × 23 u + 1 × 35.5 u = 58.5 u
The section also emphasizes the importance of understanding both molecular mass and formula unit mass in the context of chemical reactions, as they provide vital information regarding the composition and reactive proportions of substances.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Formula Unit Mass
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The formula unit mass of a substance is a sum of the atomic masses of all atoms in a formula unit of a compound. Formula unit mass is calculated in the same manner as we calculate molecular mass.
Detailed Explanation
Formula unit mass is akin to molecular mass but pertains to ionic compounds where we refer to their constituent particles as formula units instead of molecules. To find the formula unit mass, you add up the atomic masses of each atom present in the compound's formula.
Examples & Analogies
Imagine you are making a fruit salad. Each type of fruit has a certain weight. To find out how much your fruit salad weighs, you would weigh each type of fruit and then add the weights together. Similarly, calculating the formula unit mass involves adding the weights (atomic masses) of all the 'fruits' (atoms) in your 'salad' (compound).
Example of Calculating Formula Unit Mass
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For example, sodium chloride as discussed above, has a formula unit NaCl. Its formula unit mass can be calculated as– 1 × 23 + 1 × 35.5 = 58.5 u.
Detailed Explanation
To calculate the formula unit mass of sodium chloride, we need to look at its formula unit, NaCl. Sodium (Na) has an atomic mass of approximately 23 u, and chlorine (Cl) has an atomic mass of approximately 35.5 u. By adding these two values (23 u for sodium and 35.5 u for chlorine), we get a total formula unit mass of 58.5 u. This value helps us understand how heavy the formula unit of sodium chloride is compared to a standardized scale.
Examples & Analogies
Think of formula unit mass like the total cost of ingredients when cooking. If you buy 1 kg of rice for $2 and 0.5 kg of chicken for $3, the total cost can be thought of as the sum of the costs of rice and chicken. Just like totaling costs, in chemistry, we sum up atomic masses to find the formula unit mass.
Example of Calculating Formula Unit Mass for Calcium Chloride
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Example 3.2: Calculate the formula unit mass of CaCl2. Atomic mass of Ca + (2 × atomic mass of Cl) = 40 + 2 × 35.5 = 40 + 71 = 111 u.
Detailed Explanation
For calcium chloride (CaCl2), first, we note the formula indicates there are two chlorine atoms. The atomic mass of calcium (Ca) is about 40 u. Each chloride ion has an atomic mass of 35.5 u. Thus, the calculation involves the atomic mass of calcium plus twice the atomic mass of chlorine. Therefore, it’s calculated as 40 + (2 × 35.5) which equals 111 u.
Examples & Analogies
Consider making a double batch of cookies. If one batch requires 2 cups of flour (about 10 oz) and you want to double it, you will need 4 cups (or 20 oz). Similarly, in calculating the formula unit mass, when you have multiple atoms of a particular type, you 'double' their contributions (atomic masses) to find the total mass of the compound.
Key Concepts
-
Formula Unit Mass: It is the sum of the atomic masses in a formula unit of an ionic compound.
-
Ionic Compounds: Formed from positive and negative ions, represented by their formula units.
-
Molecular Mass vs. Formula Unit Mass: Molecules refer to covalent compounds, while formula units refer to ionic compounds.
Examples & Applications
Formula for sodium chloride (NaCl) has a formula unit mass of 58.5 u, calculated as (23 u + 35.5 u).
The formula unit mass of calcium chloride (CaCl2) is 111 u, calculated as (40 u + 2 × 35.5 u).
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In a bond that's ionic, you'll need to calculate, this mass that's formula, it's really first-rate!
Stories
Imagine a chef measuring ingredients for a cake. Each ingredient's weight contributes to the total formula unit mass, making the perfect recipe.
Memory Tools
To remember, think 'FUM' – Formula Unit Mass equals the Sum of atomic weights.
Acronyms
FUM
Formula Unit Mass helps weigh Ionic Compounds!
Flash Cards
Glossary
- Formula Unit Mass
The sum of the atomic masses of all atoms in a formula unit of a compound, typically for ionic compounds.
- Ionic Compound
A compound composed of ions held together by electrostatic forces known as ionic bonding.
- Molecular Mass
The sum of the atomic masses of all atoms in a molecule.
Reference links
Supplementary resources to enhance your learning experience.
