FORMULAE OF SIMPLE COMPOUNDS
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Binary Compounds
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to talk about binary compounds. These are the simplest compounds made of two different elements. Can anyone tell me what they think a compound is?

Isn't it something made up of two or more elements?

Exactly! Binary compounds consist of just two elements, like water, which is made of hydrogen and oxygen. Does anyone know how we represent these compounds?

By writing their chemical formula!

That's right! The chemical formula shows the elements and the ratio in which they combine. Let's explore how to write these formulae.
Understanding Valency
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To write the formula of a compound, understanding valency is key. Valency tells us how many electrons an atom can donate or accept. What do you think the valency of hydrogen is?

Isn't it 1?

Correct! Hydrogen has a valency of 1. What about oxygen?

Oxygen has a valency of 2!

Great! This means that in a compound like water, the combination needs to reflect these valencies. So, if we combine them, how would we write that?

It would be H2O because we need two hydrogens to balance one oxygen!

Absolutely right! This balancing is essential for creating the correct formula.
Writing Chemical Formulas
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's practice writing chemical formulas! Remember, we need to consider the valencies. If we take sodium and chlorine, could anyone tell me their valencies?

Sodium has a valency of 1 and chlorine has a valency of 1 too!

Perfect! How do we combine them to write the formula for sodium chloride?

It would just be NaCl since both valencies are 1.

Exactly! Now, how about magnesium and oxygen?

Magnesium has a valency of 2 and oxygen has a valency of 2.

So the formula would be MgO.

Close, but remember, since magnesium is 2 and oxygen is 2, we still write it as MgO without needing to change the subscript. Excellent work!
Complex Compounds and Polyatomic Ions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's move on to some more complex compounds. What happens when we deal with polyatomic ions?

We have to treat the group as a single unit?

Yes! For example, in calcium nitrate, you have calcium, which has a valency of 2, and the nitrate ion has a valency of 1. How would we write the formula for calcium nitrate?

It would be Ca(NO3)2 because we need two nitrate ions to balance the charge of one calcium ion!

Exactly! Great teamwork! Keep practicing these rules, and writing chemical formulas will become second nature for you.
Summary of Key Points
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's wrap up what we've learned about binary compounds and their formulas. Can someone summarize the steps to write a chemical formula?

First, identify the elements and their valencies!

Then, cross the valencies and ensure the overall charge is balanced!

Finally, we can write the formula using the symbols and subscripts.

Excellent summary! Remember, practice makes perfect, and these foundational concepts are crucial for your chemistry studies. Keep reviewing and practicing!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, students learn about binary compounds, which consist of two different elements. It covers writing chemical formulae based on the valencies of the constituent elements and provides examples to illustrate the concepts.
Detailed
Formulae of Simple Compounds
In chemistry, binary compounds are the simplest types of compounds made up of only two different elements. Writing the chemical formula of a compound involves understanding the valency of each element involved in the combination. Valency refers to the combining capacity of an element.
To write a chemical formula:
1. Identify the symbols of the elements involved.
2. Determine the valency of each element.
3. Cross over the valencies to balance the charges, ensuring the overall compound is neutral.
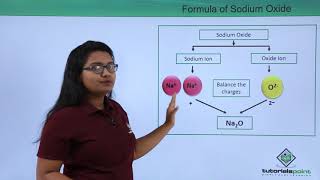
For example, the formula for hydrogen chloride is HCl. In this case, hydrogen (H) has a valency of 1, and chlorine (Cl) also has a valency of 1, so they combine in a 1:1 ratio. The same principle applies to more complex compounds, such as sodium carbonate (Na2CO3) where sodium's valency is 1 and carbonate has a valency of 2, resulting in two sodium ions to balance the charge of one carbonate ion.
Importance
Writing chemical formulae correctly is crucial in understanding chemical reactions and stoichiometry in chemistry.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Binary Compounds
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The simplest compounds, which are made up of two different elements are called binary compounds.
Detailed Explanation
Binary compounds are basic compounds that consist of exactly two different elements. This means the substance is formed when two different types of atoms unite together. A common example of a binary compound is water, which is made up of two elements: hydrogen and oxygen. The chemical formula for water is H₂O, indicating two hydrogen atoms bonded to one oxygen atom.
Examples & Analogies
Think of binary compounds like securing two ropes together to make a longer rope. Each rope represents a different element, and when you tie them together, they form a new, stronger 'compound' rope that can be used for more complex tasks.
Valency and Chemical Formulae
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
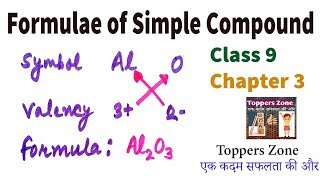
While writing the chemical formulae for compounds, we write the constituent elements and their valencies as shown below. Then we must crossover the valencies of the combining atoms.
Detailed Explanation
When creating a chemical formula, understanding the concept of valency is crucial. Valency refers to the combining capacity of an element, which essentially tells us how many atoms of one element can bond with atoms of another element. For example, if one element has a valency of +2 and another has a valency of -1, the resulting compound will be formed by crisscrossing these valencies. This means we would need two of the -1 charged atoms to balance out the one +2 charged atom.
Examples & Analogies
Imagine two people meeting at a party. One is a strong person who can lift the weight of two people (valency of +2), and the other is light, needing support from just one person (valency -1). To keep everything balanced, the stronger person teams up with two lighter people to make a balanced group. Similarly, in chemistry, elements team up to form stable compounds.
Examples of Writing Chemical Formulae
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
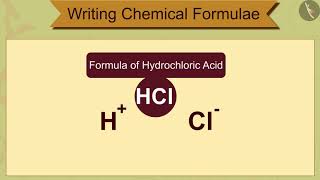
Some examples: 1. Formula of hydrogen chloride: HCl. 2. Formula of hydrogen sulphide: H₂S. 3. Formula of sodium nitrate: NaNO₃.
Detailed Explanation
Writing chemical formulae involves knowing the elements involved and their respective valencies. For instance, for hydrogen chloride (HCl), hydrogen has a valency of +1 and chlorine has a valency of -1, so they balance each other perfectly without any need for crisscrossing. For hydrogen sulphide (H₂S), hydrogen's valency of +1 means we need two hydrogen atoms to balance the single sulphide atom with a -2 valency. In sodium nitrate (NaNO₃), sodium has a +1 valency, while the nitrate ion (NO₃) has a -1 charge. Thus, the structure is straightforward based on the elemental valencies.
Examples & Analogies
Think of these chemical formulae as recipes. Just as a recipe outlines the exact amounts (quantities) of ingredients needed to make a dish, the chemical formula specifies how many atoms of each element are needed to create a compound. For instance, to make lemonade, you need one cup of lemon juice and three cups of water, similar to needing one Na atom and three oxygen atoms in a compound formula.
Key Concepts
-
Binary Compounds: Compounds made of two different elements.
-
Valency: The combining capacity of an element.
-
Chemical Formula: A symbolic representation showing elements and their quantities.
-
Polyatomic Ions: Charged groups of covalently bonded atoms that behave as a single unit.
Examples & Applications
Example of water as a binary compound: H2O.
Example of sodium chloride: NaCl.
Example of calcium nitrate: Ca(NO3)2.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
If H and Cl make a pair, it's HCl, beyond compare!
Stories
Once upon a time, in a lab far away, H and Cl met and decided to bond. Together they formed HCl, a story of unity in diversity!
Memory Tools
B for Binary, C for Compound - remember that they are two elements bound!
Acronyms
BCE
Binary Compounds - Elements!
Flash Cards
Glossary
- Chemical Formula
A symbolic representation of the composition of a compound that indicates the elements present and the number of atoms of each.
- Binary Compound
A chemical compound consisting of two different elements.
- Valency
The combining capacity of an element, indicating how many electrons it can gain, lose, or share.
- Polyatomic Ion
An ion composed of two or more atoms that are covalently bonded and that functions as a single ion.
Reference links
Supplementary resources to enhance your learning experience.
